BioSig Technologies, Inc. (BSGM) is a Minnesota-based medical device company developing a proprietary technology platform designed to improve the rapidly growing multi-billion dollar electrophysiology (EP) marketplace. The company’s leading product is Pure EP™, a surface electrocardiogram (EKG) and intracardiac multichannel recording and analysis system designed to assist electrophysiologists during catheter ablation procedures to treat cardiac arrhythmia. Pure EP is comprised of proprietary hardware and software that acquires and displays high-fidelity cardiac signal recordings. By providing precise, uninterrupted, real-time evaluations of EKG and electrogram data, Pure EP improves the clinical decision-making process by the electrophysiologists in the EP lab. Quite simply, Pure EP results in better cardiac mapping and ablation point targeting, which can lead to reduced cost and superior outcomes for patients with arrhythmia.
Below is an introduction to BioSig Technologies and a teach-in on the EP market, including a review of cardiac arrhythmia and ablation procedures. I see the company as incredibly undervalued based on the expected growth in the EP market over the next decade, and believe that the company’s superior technology platform is a credible threat in an industry segment dominated by some of the world’s largest players, including Boston Scientific, St. Jude Medical, Abbott Labs, Johnson & Johnson, and GE Healthcare; all of which have a history of acquiring smaller EP players with potential disruptive technology. Beyond this potential obvious exit strategy, BioSig has partnered with some of the country’s leading cardiac centers, including the Texas Cardiac Arrhythmia Institute, UCLA Cardiac Arrhythmia Center, U.H. Case Medical Center in Cleveland, William Beaumont Hospital in Michigan, Mount Sinai Medical Center in NY, and the Mayo Clinic in Minnesota to generate clinical data and validate proof-of-concept with Pure EP. Finally, the company is run by what looks to be an outstanding leadership team of experienced industry executives, renowned cardiac electrophysiologists, and astute entrepreneurs.
An Introduction to Electrophysiology
Electrophysiology is the study of the electrical activity of the heart. An electrophysiology study (EPS) is a diagnostic procedure performed by a specialized cardiologist, called an electrophysiologist, with the intent to help understand the nature of an abnormal heart rhythm. The results of an EPS facilitate the assessment of complex arrhythmias, elucidate symptoms, evaluate abnormal EKGs, assess the risk of developing arrhythmias in the future, and design treatment options, which may include pharmaceutical therapy or the surgical implantation of a cardiac defibrillator, pacemaker, or a cardiac ablation procedure. An EPS takes place in an electrophysiology or catheterization (cath) lab, usually under mild sedation.
The primary diagnostic output during an electrophysiology study is an electrogram, or intracardiac signal. In essence, an EPS is like an EKG performed from inside the heart through the use of specialized catheters inserted into the femoral artery or vein through small incisions in the inner thigh and guided by x-ray videography into the heart. The electrophysiologist then sends small electric pulses through these catheters (often multiple catheters are used) to make the heart beat at different speeds. Electrical signals produced by the heart are picked up by the catheters and recorded. An EPS can last several hours, during which time an immense amount of information is recorded and analyzed. This is called cardiac mapping, and the procedure allows the electrophysiologist to locate where arrhythmias are coming from within the heart or aorta.
Cardiac Arrhythmia
The human heart is made up of four chambers, two upper chambers called atria and two lower chambers called ventricles. The heart also has four valves that open and close to let blood flow in only one direction when the heart beats normally. The four heart valves are the tricuspid valve, located between the right atrium and right ventricle, the pulmonic valve, between the right ventricle and the pulmonary artery, the mitral or bicuspid valve, between the left atrium and left ventricle, and the aortic valve, between the left ventricle and the aorta. Each valve has a set of flaps. The bicuspid valve has two flaps; the others have three. Blood flow occurs only when there’s a difference in pressure across the valves, which causes them to open.
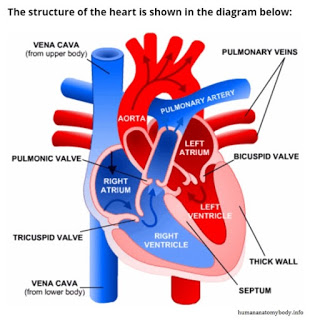
An arrhythmia is an abnormal heartbeat or a change from the normal sequence of electrical impulses that control the beating (contraction) of the heart. The beating of the heart is caused by an electrical impulse that begins in the sinoatrial node (also called the SA node or sinus node), and moves from the right atrium throughout the atria to the atrioventricular (AV) node. From the AV node, electrical impulses travel down a group of specialized fibers called the His-Purkinje system to all parts of the ventricles. This is the exact route that must be followed for the heart to pump in a synchronous matter causing blood to flow through the valves in the proper direction. As long as the electrical impulse is transmitted normally, the heart pumps and beats at a regular pace.
An arrhythmia occurs when the heart beats outside of a normal pace, which may include beating too fast or too slow, or in an irregular fashion. There are three main types of arrhythmia:
· Tachycardia – An abnormally fast heartbeat (usually above 100 beats per minute) at resting rate.
· Bradycardia – An abnormally slow heartbeat (usually less than 40 beats per minute) at resting rate.
· Fibrillation – An irregular beat of the heart that may include tachycardia or bradycardia, or the heart beating in a chaotic or quivering fashion.
Chronic tachycardia, i.e. not brought on by temporary emotional, chemical, or physical stress, reduces the efficiency of the heart and can lead to dizziness, shortness of breath, heart palpitations, angina, and syncope. Tachycardia can originate in a narrow complex, such as within the sino-atrial (SA) node near the base of the superior vena cava (sinus tachycardia) or within the atrium (atrial tachycardia), or within the more wide complex of the ventricles (ventricular tachycardia). Similarly, bradycardia can also result in dizziness or syncope, and patients may experience fatigue and in extreme cases, cardiac arrest. Bradycardia can be brought on by coronary artery disease, endocarditis, myocarditis, hypothyroidism, electrolyte imbalance, or myocardial infarction.
Atrial fibrillation (AF) is the most common form of arrhythmia. The rapid, abnormal firing of electrical impulses causes the atria to quiver or beat in a chaotic (irregular) fashion which causes the ventricles to pump the blood ineffectively throughout the body. According to the American Heart Association (AHA), an estimated 2.7 million Americans are living with AF. Untreated AF doubles the risk of cardiovascular-related death and causes a 4-5-fold increase in the risk of stroke (source: AHA). According to the U.S. Center for Disease Control and Prevention (CDC), more than 750,000 hospitalizations and 130,000 deaths occur each year in the U.S. because of AF. AF is the underlying cause for 15-20% of ischemic strokes and costs the U.S. an estimated $6 billion in direct medical expense each year.
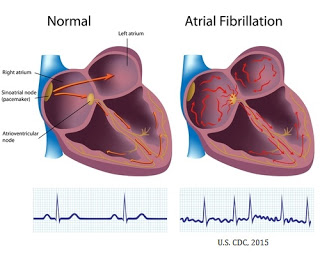
The primary treatment options for AF include procedures designed to control the heart’s rhythm and rate, medications to reduce the workload of the heart, and/or medications to prevent the formation of clots to reduce the risk of stroke. Electrical cardioversion is a medical procedure where an electrical shock is delivered to the heart through paddles or patches placed on the chest that shock-stop the heart momentarily in hopes that when it resumes the rhythm is normal. Pharmaceutical options for the treatment of AF include antiarrhythmic drugs, calcium channel blockers, beta-blockers, and antiplatelet and/or anticoagulant medications to thin the blood and reduce the risk of clot formation.
For many patients, pharmaceutical options are not sufficient to control the arrhythmia. In these patients, pacemakers or cardiac defibrillators may be surgically implanted to provide low-energy pulses to the heart to restore rhythm and monitor the heart to prevent sudden cardiac arrest. In the U.S., over 225,000 pacemakers and 135,000 cardiac defibrillators are implanted each year.
Catheter Ablation Therapy
Medications or cardioversion to control atrial fibrillation is not always effective. In these cases, a cardiologist may perform a procedure to ablate the area of heart tissue that’s causing the erratic electrical signals in an effort to restore the heart to a normal rhythm. This is done by the use of specialized catheters that deliver radiofrequency energy, extreme cold (cryotherapy), or heat to ablate small areas of the heart, either by isolating or destroying the tissue causing the asynchronous signal. This corrects the arrhythmia without the need for medications or implantable devices. However, determining the exact tissue to isolate that is causing the erratic electrical signals is paramount to a successful catheter ablation procedure, hence the importance of the cardiac mapping and real-time information noted above during the electrophysiology study.
Highly fidelity 3D cardiac mapping can only be obtained through the use of a cutting-edge diagnostic software and hardware system employed by the electrophysiologist during the EPS. The system helps the electrophysiologist in locating the defective tissue and in monitoring target ablation sites in real-time.
A cardiac mapping system with low fidelity or high signal-to-noise ratio can result in incomplete ablation of the target area, which results in recurring arrhythmia and the potential for a repeat procedure at some point in the future. A poor signal quality might also lead to longer procedure time, adding cost to the hospital and potential detrimental adverse effects on the patient. For example, recent data published in the Journal of the American Medical Association (JAMA) found that radiofrequency catheter ablation procedures had a success rate of only 46% on the first try. In fact, 47% of the study population had symptomatic recurrences of atrial tachyarrhythmia and declining quality of life measures within two years of the procedure (Morillo et al., 2014). The website www.stopafib.org reports success rates for catheter ablation procedures, usually in concert with anti-arrhythmia medications, ranges between 30 and 60% after a single procedure.
An analysis of patient outcomes following catheter ablation procedures for the treatment of AF published in Arrhythmia and Electrophysiology (Cappato et al., 2009) found that the average patients undergoes 1.3 procedures before the success rate, defined as no required further anti-arrhythmic medication, eclipsed 80%. Ineffective technique was the primary cause of recurrent arrhythmia necessitating a follow-up procedure. The study looked at 16,309 patients from 521 centers in 24 countries around the globe. To further this point, Dr. John Mandrola MD, a cardiac electrophysiologist in private practice and columnist at theHeart.org / Medscape stated in November 2012:
“One of the greatest drawbacks of AF ablation is the need to redo the procedure. Recurrent AF occurs in too many ablation patients—up to 30% to 40% in honest reports. In nearly all redo procedures, the problem is gaps in conduction block between the pulmonary vein and left atrium. Again, it’s not exactly clear why these gaps occur, though lack of transmural burns is the leading hypothesis. Good AF ablationists understand the importance of making each burn effective and continuous, but the technology is not quite there yet. It’s hard to make continuous lines with dots.”
How Pure EP Can Change The Game
As Dr. Mandrola noted above, the less than desirable success rate of catheter ablation procedures can be directly tied to the limits of technology. The electrophysiology lab environment and recording systems create significant amounts of noise and artifacts during procedures which hamper recordings of small electrophysiological potentials. Preserving spatiotemporal (space and time) characteristics of the signal in a challenging recording environment is a difficult task. Eliminating the noise and artifact is a significant challenge when setting up an EP lab, and is not always successful. To remove noise and artifacts, recording systems that are currently on the market offer a family of low pass, high pass, and notch filters, however, these filters may alter signal information context.
High-quality information provided by the recording system is essential for an electrophysiologist to determine ablation strategies during the termination of various arrhythmias. Therefore, it is important that the recording system’s noise removal technique does not alter appearance and fidelity of these potentials. BioSig’s PURE EP™ System is a novel hardware and software platform designed to obtain and display important clinical data during electrophysiology procedures. Pure EP delivers precise and uninterrupted evaluations of electrocardiograms and electrograms to assist the electrophysiologist in real-time during the ablation procedure.
And importantly, Pure EP is designed to be used with existing EP recording devices, essentially creating a new category within the market where cath labs can add on the Pure EP system without the significant cost of replacing or upgrading the significant and expense technology already in place. It is designed to improve the signal and recording so that analysis at previously undetectable levels can be conducted with high accuracy. This is the key to the BioSig investment thesis, as the company is not necessary competing with industry behemoths Boston Scientific, St. Jude Medical, Abbott Labs, Johnson & Johnson, and GE Healthcare, but instead bringing a new product into the market – and EP Information System – that makes existing platforms better.
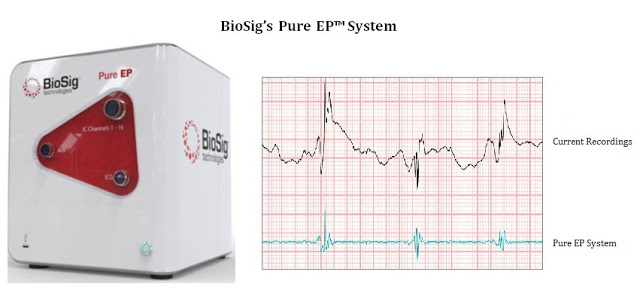
In the next few months, BioSig should be presenting data on the Pure EP system at major medical conferences. For example, preclinical data gathered during a series of studies from 2015 at the Mayo Clinic was presented at the 13th International Dead Sea Symposium on Innovations in Cardiac Arrhythmias and Device Therapy earlier in March 2016. I expect management to make this data available to the public in the next few weeks. Also expected in the next few weeks is data in a professional engineering association publication. I anticipate that once the market understands the level of improvement in data analysis that can be obtained through Pure EP, physician and investor interest in BioSig Technologies will soar.
The EP Market Opportunity
Pure EP is a Class II medical device, and thus can gain U.S. FDA approval through a 510(k) application. This means the company does not need human clinical data to submit an application to the agency. Proof-of-concept validation with Pure EP has already been achieved through the company’s collaboration with UCLA Labs. As noted above, preclinical work was conducted at the Mayo Clinic; and the company is collaborating with several other leading institutions around the U.S. Finally, BioSig has already initiated a technology development and manufacturing agreement with Minnetronix, an FDA registered and ISO Certified medical technology and innovation company with deep expertise in electronic and electromechanical devices. Management plans to file the 510(k) application late 2016 or early 2017. The U.S. FDA is expected to make a decision 3-6 months later, which should put the company in the position to enter the large and rapidly growing EP market by the middle of 2017. CE Mark in Europe should follow in 2018.
As noted above, According to the AHA, an estimated 2.7 million Americans are living with AF. Due to the aging population, the number is expected to eclipse 8 million by 2050. Untreated AF doubles the risk of cardiovascular-related death and causes a 4-5-fold increase in the risk of stroke (source: AHA). According to the U.S. Center for Disease Control and Prevention (CDC), more than 750,000 hospitalizations and 130,000 deaths occur each year in the U.S. because of AF. Ventricular tachycardia accounts for over 350,000 sudden cardiac deaths in the U.S. each year and AF is the underlying cause for 15-20% of ischemic strokes. This costs the U.S. an estimated $6 billion in direct medical expense each year; indirect costs total another $20 billion.
According to Global Industry Analysis, the electrophysiology device market will grow at a 12.1% compound annual growth rate, from $2.5 billion in 2012 to $5.5 billion by 2019, making it one of the fastest growing medical device segments. Accordingly, the number of catheter ablation procedures done in the U.S. is expected to grow at 10.5% CAGR between 2012 and 2017. In 2014, there were a total of 260,000 and 600,000 catheter ablation procedures done in the U.S. and worldwide, respectively. Approximately half of these procedures are for atrial fibrillation or ventricular tachycardia. The average price of such a procedure ranges between $20,000 and $40,000 in the U.S.
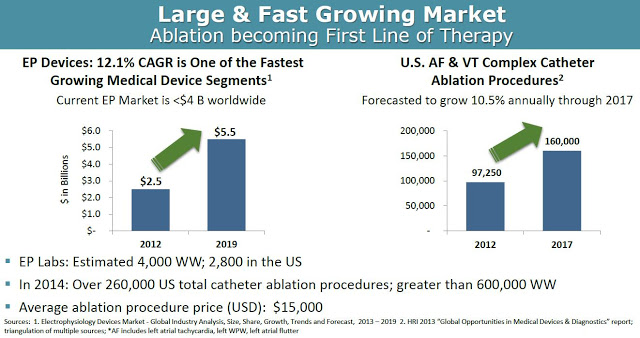
From a market penetration standpoint, BioSig could see significant uptake of the Pure EP device upon commercialization in 2017. As noted above, Pure EP is designed to deliver improved signals and information over the existing already installed equipment, making the product a nice add-on to the incredibly expensive systems (~ $250,000 per install) in place at EP labs across the world. There are a growing number of EP labs in the world, with an estimated 3,500 in the U.S. and another 2,000 outside the U.S. It’s a relatively straightforward pitch for BioSig, or a potential commercial partner like Boston Scientific, St. Jude, or General Electric that already have sizable market share and a vested interest in keeping those very important working relationships with cardiac electrophysiologists.
In the coming months, I will provide a detailed revenue model on BioSig showing a projected ramp in revenues from the launch of Pure EP around the middle of 2017. However, I see Pure EP as a $615 million cumulative revenue opportunity over the next 10-15 years. This is based on a best-guess sales price of $175,000 per install and a 25-30% penetration of the estimated 5,500 EP cath labs in the developed world. I also expect that BioSig will offer both software and hardware upgrades to the installed system, with a useful life of roughly 10 years, along with yearly service revenue that sums to total revenue of around $405,000 per system installed over a 10 year period. With a 15% discount rate, I estimate the net present value of an install to be roughly $250,000.
Major Collaborators & Experience Management
The obvious exit strategy for BioSig is to sell to one of the industry behemoths, Boston Scientific, St. Jude, General Electric, or Johnson & Johnson. Deals have been plentiful in this space over the past few years. For example, the deal that Abbott Labs did in October 2014 to acquire privately-held Topera, Inc. for $250 million is pretty representative of the upside potential for BioSig. Topera developed a novel diagnostic catheter and mapping software, or rotor identification system, which help electrophysiologists identify and target the specific areas of a person’s heart that are perpetuating AF. Other important M&A transactions in this space can be seen in the chart below, with an average deal price of $275 million (source: BioSig March 2016 Investor Presentation).
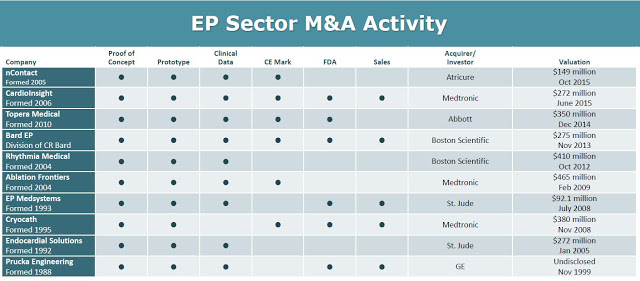
Besides having a quality product, an important variable for any small-cap company when dealing with potential large-cap suitors is industry credibility. In this regard, it is important to note that BioSig has partnered with some of the country’s leading cardiac centers, including the Texas Cardiac Arrhythmia Institute, UCLA Cardiac Arrhythmia Center, U.H. Case Medical Center in Cleveland, William Beaumont Hospital in Michigan, Mount Sinai Medical Center in NY, and the Mayo Clinic in Minnesota on the development and preclinical / clinical testing of the Pure EP system. In fact, on March 23, 2016, BioSign announced the establishment of an advanced research program with the Mayo Clinic to further the development of the platform to treat complex arrhythmias. These relationships are incredibly important, not only for concept validation of Pure EP, but also once the company initiates commercial sales activities.

Management credibility and execution is the final piece of the puzzle. BioSig looks to be run by an outstanding team of experienced industry executives, renowned cardiac electrophysiologists, and astute entrepreneurs. The management team and Board of Directors is a mix of industry and Wall Street, and the company’s scientific advisory board includes directors and professors some of the nation’s top cardiology research centers.


Conclusion
BioSig Technologies has a unique opportunity to provide a product that can deliver a meaningful improvement in diagnostic effect and therapeutic outcome in the large and rapidly growing EP market. I like the investment story here because Pure EP has the potential to be a highly complementary product to an existing installed base that is dominated by industry behemoths. BioSig is developing a credible threat in this space, and that will likely result in a highly lucrative end-game scenario for shareholders. Pending data from highly-respected sources in the next several months, along with progress towards commercialization should result in an increase in market value for the stock. Management has also spoken publicly about pursuing an uplist of the common stock to the NASDAQ market in the coming months. I believe this will help solve liquidity and visibility issues currently keeping the stock price at an artificially low level.
From a valuation standpoint, BioSig is an estimated 18 months away from revenue generation, with peak sales likely five years later based on Pure EP system installs. Above I outlined my belief that Pure EP offers a $615 million cumulative revenue opportunity once launched. Revenue in year five is likely $150 million based on peak system install penetration, with subsequent revenues booked over the next decade following thanks to both hardware and software upgrades and yearly service contracts.
Large medical device players like Medtronic, Boston Scientific, St. Jude, J&J, and Abbott Labs trade at approximately 3.7x projected revenues and 13.6x projected EBITDA (see below).
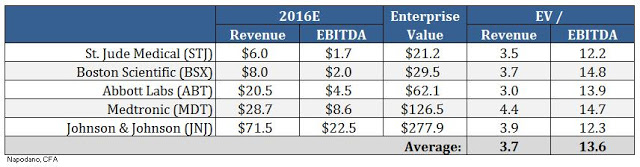
If we apply the industry average EV/Rev multiple to our projected peak sales estimate of $150 million in 2022, and then discount back to present day at 15%, we arrive at a fair market value for BioSig of $182 million. If we assume the industry average EBITDA margin of 28% and apply a similar valuation methodology, only this time using EV/EBITDA multiples, we arrive at a fair market value for BioSig of $186 million. The blended average of these two methods yields a target market value of $185 million. This equates to $10.65 per share on a basic count and $7.55 per share on a diluted basis assuming about one-third of the outstanding stock options and 50% of the outstanding warrants become exercised and turned into common shares.
This target is calculated by applying multiples derived from the industry’s largest players with mature businesses and significantly lower growth rates. It is highly plausible that a company like St. Jude, Abbott, J&J, or Boston Scientific would pay significantly higher than 3.7x peak sales or 13.6x peak EBITDA. After all, Pure EP would be plugged into the existing business at these companies, likely resulting in significantly higher profitability than what a small, standalone company like BioSig could achieve. This is also a fiercely competitive space where 1% market share within the cath lab is worth $30 million. A complementary “bolt-on” type product like Pure EP would be highly coveted and too important to allow falling into a competitor’s hands.
I’m also likely underestimating the profitability of software upgrades and yearly service contract revenue. In the coming weeks I intend to publish a more detailed model for investors; but, as noted above, I’m waiting for the release of the preclinical and clinical data expected shortly. Nevertheless, with some conservative assumptions, I believe BioSig Technologies Inc. represents a compelling investment story given the current market value of only $22 million for the basic common stock.


