Acute Leukemia – A Quick Overview
Leukemias are cancers of the blood and involve bone marrow, circulating white blood cells, and organs such as the spleen and lymph nodes. The cancer usually manifests in the bone marrow and results in a high number of abnormal white blood cells called blasts (also called leukemia cells). The presence of these immature (not fully developed) blast cells can lead to a number of physiological symptoms such as bleeding, bruising, anemia, fatigue, and an increased risk of infection. The symptoms get worse as the number of blast cells increase and subsequently reduce the number of healthy normal blood cells through a crowding out effect.
Acute leukemia refers to malignancies that occur earlier in the differentiation and maturation process for hematopoietic stem cells (HSCs). HSCs are multipotent cells that give rise to common myeloid or common lymphoid progenitor cells that go on to form the specialized cells found in human blood, including monocytes, macrophages, neutrophils, basophils, eosinophils, erythrocytes, dendritic cells, megakaryocytes, platelets, granular lymphocytes, T lymphocytes, and B lymphocytes.
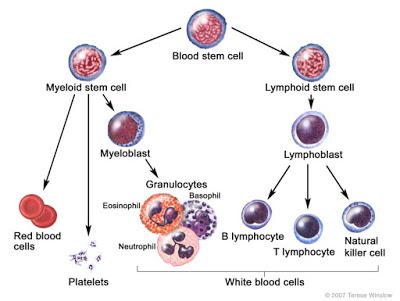
Malignancies that occur in immature myeloid progenitor cells are called Acute Myeloid Leukemias (AML), whereas malignancies that occur in immature lymphoid progenitor cells are called Acute Lymphoblastic Leukemias (ALL). Acute leukemias are rapid growth (quick onset) diseases. Chronic Myeloid Leukemia (CML) and Chronic Lymphoblastic Leukemia (CLL) are cancers that occur later in the maturation and differentiation process, and tend to be slower growing diseases.
There are several types of AML depending on where the arrested development occurs in the cell lineage. For example, a malignancy that occurs in monoblasts is called Acute Monoblastic Leukemia, whereas a malignancy that occurs in megakaryoblasts and erythroblasts are called Acute Megakaryocytic and Acute Erythrocytic Leukemia, respectively. Regardless of where the malignancy occurs with respect to cell lineage, the symptoms for the different types of AML are generally pretty similar and result from an increase in immature blast cells and reduced production of healthy normal specialized cells. More specifically, as the number of blasts increase, the number of specialized cells, including red blood cells, platelets, and white blood cells decrease, resulting in symptoms such as anemia, bleeding, and risk of infection.
Diagnosis for AML is generally done through analysis of a complete blood count (CBC), specifically looking for the causes of the anemia, thrombocytopenia, or leukopenia. Confirmation of leukemia is accomplished by analysis of bone marrow aspirate looking at the number of immature blast cells as a percent of the total cell count. One is generally considered to have AML when the number of immature myeloid blast cells reaches 20% of the total cell count. Myelodysplastic syndrome (MDS), formerly known as “Pre-Leukemia”, is diagnosed when the number of blast cells is between 5 and 20% of the total cell count, and may or may not develop into full AML. A normal, healthy individual will have 2-3% blast cells in the bone marrow.
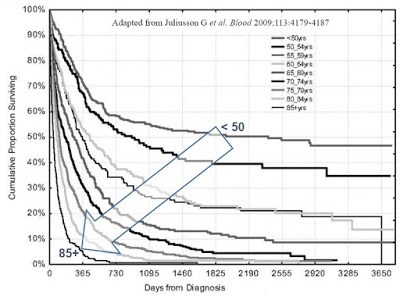
According to the U.S. National Cancer Institute (NCI), there will be estimated 20,800 new cases of AML in the U.S. in 2015. AML is the most common form of leukemia, but still only represents about 1.3% of call cancers. AML will kill roughly 10,500 individuals in 2015. The five-year survival rate is only 25.9% (NCI 2005-2011 SEER Fact Sheet). The disease is most common in the elderly, with the average age of onset approximately 67 years old, with the vast majority of patients between the ages of 55 and 75 years old (Cancer.org).
Treatment For Acute Myeloid Leukemia
Generally speaking, leukemia is treated with chemotherapy. The effects of systemic chemotherapy are most evident on rapidly dividing cells in the body, which makes treatment highly effective for acute diseases such as AML. The systemic chemotherapy is deployed in various stages, with the first stage known as the induction or “wipe out” phase, generally lasting between four to six weeks. The goal of the induction phase is to kill all the cancerous cells and put the patient into remission. This is accomplished through administration of high-dose chemotherapy. Radiation therapy may also be deployed if the patient is at risk of the cancer spreading to other organs.
The most commonly used chemotherapy regimen for AML is cytarabine, given continuously for seven days through an intravenous line, and an anthracycline drug, such as daunorubicin or idarubicin, given in a single intravenous dose for the first three days of treatment. This is sometimes known as the “7+3” regimen. These drugs kill AML cells over the first 7 to 14 days; it then takes the normal bone marrow about 14 days to recover and produce normal blood cells again. According to the Cleveland Clinic, complete response rates for 7+3 induction range between 70 and 80%, with a median duration of remission of 12 to 18 months and 50% overall survival rate at five years for those in complete remission after the induction phase (Champlin R, et al., 1987). Overall survival rate and patient outcome is highly correlated with age of disease onset.
Unfortunate side effects of this type of high-dose chemotherapy include hair loss and gastrointestinal tract disorders such as nausea, vomiting, diarrhea, and fatigue. Treatment-related mortality is significantly higher for patients above the age of 60 years old, and can range as high as 30% (Cleveland Clinic). High doses of cytarabine can also be associated with cerebellar and ophthalmologic toxicity, particularly in patients over the age of 60 years. Anthracycline has been associated with significant cardiotoxicity and is contraindicated for use in patients with congestive heart failure, coronary artery disease, ischemia, and hypertension (Volkova M, et al., 2011).
The next phase is known as consolidation phase. The purpose of consolidation is to keep the patient in remission by killing all remaining leukemia cells to prevent relapse. Consolidation also helps prevent the spread of the cancerous cells to other organs, including the brain. Consolidation phase chemotherapy includes additional rounds of high-dose cytarabine (HiDAC) for patients younger than 60 years. HiDAC yields a 4-year disease-free survival rate of 50±15%, but carries with it a 5% treatment-related mortality (Schiller G., et al., 1992). Patients over the age of 60 have significantly lower outcomes based on co-morbidity of cardiovascular disease and significantly higher incidence of treatment-related mortality, although the statistics are improving thanks to less intense treatment regimens (Othus M, et al., 2013).
Consolidation is followed by the maintenance phase, in which low-dose chemotherapy is administered over the course of two to three years. Patients diagnosed with AML at younger ages have much higher long-term survival expectations than elderly individuals. Data published by Pulte D., et al., 2009 shows 5- and 10-year relative survival estimates of 21.4% and 18.7% for all ages combined, 62.2% and 57.4% for ages 25–34, and 60.6% and 58.1% for ages 35–44, versus survival rates of only 30% for patients older than 60 years (Garvin, 2012).
For patients in remission with high risk of relapse or who are refractory to HiDAC + anthracycline, allogeneic hematopoietic stem cell transplantation (HSCT) remains the only option. HSCT, or the simpler term of bone marrow transplant (BMT), does provide potential for a curative treatment, but carries significant logistical and safety risks, including finding a matched donor and keeping the patient infection free during the regrowth phase, as well as reducing risk of rejection or graft-vs-host disease (GvHD).
Traditionally, patients are prepared for transplantation with intensive preparatory therapy. The two most widely used regimens consist of cyclophosphamide and large doses of fractionated total body irradiation (TBI) or busulfan and cyclophosphamide. These regimens effectively eliminate nearly all normal and malignant marrow cells and produce sufficient immunosuppression to prevent rejection of allogeneic hematopoietic cells (Jones, CV, et al, 2009).
BMT Conditioning – A Delicate Balance
Prior to receiving a bone marrow transplant, conditioning regimens are deployed to ablate the bone marrow in effort to wipe out the mutated or malignant cells and provide immunosuppression to prevent rejection of the transplanted graft. This is a critical element in the BMT procedure as it is important to deliver a substantial enough dose of the cytotoxic agent to effectively destroy all the bone marrow cells and reduce the risk of GvHD, while maintaining a level of tolerability as to not kill the patient. This can be accomplished through the use of chemotherapy or radiation therapy. Commonly used chemotherapeutic agents include busulfan, cyclophosphamide, and fludarabine. Each of these agents has significant side effects, with GI toxicity, hair loss, nephrotoxicity, hepatotoxicity, neurotoxicity, pulmonary, and cardiovascular toxicity being the most common.
Total body irradiation (TBI) is the use of x-rays to accomplish bone marrow ablation. The total dose of radiation usually is given over several days just prior to transplant in a radiation oncology department. Each radiation treatment typically takes 20 to 30 minutes to administer and significant precaution must be taken to assure proper dose and exposure.
The immediate side effects of radiation therapy, which occur within the first few hours and days, may include diarrhea, nausea, vomiting and tiredness. Mouth sores also can be a problem due to the combination effect of systemic chemotherapy and radiation. Starting several days after radiation therapy, hair loss, sunburn and dryness of the skin may be experienced. Potential long-term effects of systemic chemotherapy and radiation include cataracts (clouding of the lens in the eye), sterility, and the possible development of a second cancer.
The trend over the past decade or more has been to deliver reduced-intensity radiation or chemotherapy; essentially, by providing the minimum level of cytotoxic agent necessary to provide sufficient immunoablation to prevent graft rejection and reduce the tumor burden without overlapping toxicity and increasing the risk of secondary cancer or treatment-related mortality (Gyurkocza B, et al., 2014). The chart below shows the delicate balance between intensity of dose, toxicity of the regiment, and the risk of developing GvHD.
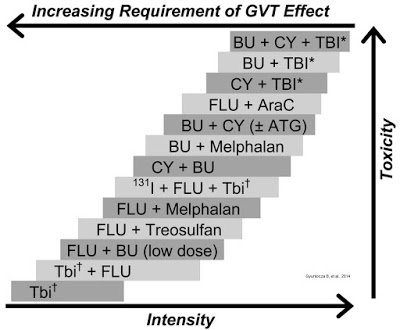
Reduced intensity conditioning (RIC), while effective at minimizing toxicity, does leave the potential open for relapse or GvHD. A large retrospective analysis conducted by Schmid C, et al., 2012 looked at the outcome of 2,815 patients that received RIC over a ten year period between 1999 and 2008. Results showed that the cumulative incidence of relapse was 32±1%, and that relapsed patients had an estimated 2-year overall survival of only 14%. The authors concluded that the relapse rates are only modestly higher than what has been seen for standard conditioning or myeloablative conditioning, and that the absence of bone marrow blasts and acute GvHD are two important prognostic indicators of survival.
Retrospective analysis published by Gupta V, et al., 2003 noted the following: “High-dose busulfan combined with cyclophosphamide (BuCy) and cyclophosphamide in combination with total body irradiation (CyTBI) are the two most commonly used conditioning regimens for AML allografts. From the available data, there are no significant differences in survival with these two regimens. A small benefit of decreased relapse rate with CyTBI is counterbalanced by a nonsignificant increase in treatment-related mortality.”
Work by Shimoni A, et al., 2006 found that RIC offers similar overall survival data to high-intensity myeloablative conditioning, 47-49% vs. 50%, but that high intensity myeloablative was associated with significantly higher non-relapse mortality, 22% vs. 8%. Another study published by Alyea E, et al., 2005 found that RIC improved overall survival after two years versus in high-intensity myeloablative conditioning, 39% vs. 29%, in patients over the age of 50 years old. Similarly, the authors found that non-relapse mortality was lower in the RIC population, 32% vs. 50%, but that the relapse rate was higher 46% vs. 30%, again confirming the small therapeutic window and delicate balance between the minimal effective dose and maximum tolerated dose for BMT conditioning.
Actinium Pharma – Two Strategies To Improve Outcomes
Actinium Pharmaceuticals (NYSEMKT:ATNM) is a biopharmaceutical company specialized in the development of cancer drugs, with a specific focus on leukemia. The company’s two most advanced product candidates are based on patented technology co-developed with Memorial Sloan Kettering Cancer Center (MSKCC) for combining the cancer targeting precision of monoclonal antibodies (mAb) and a proprietary alpha particle immunotherapy (APIT) platform. Monoclonal antibodies are genetically engineered proteins that specifically target certain cancer cells, and alpha emitting radioisotopes are unstable chemical elements that decay by releasing alpha particles that kill any cell in the immediate proximity of where they are released.
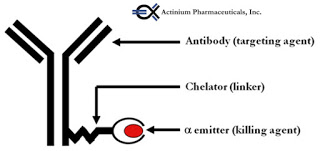
The company’s most advanced candidate is Iomab-B (BC8-I-131), a combination monoclonal antibody that targets a lymphocyte common antigen, CD45, and radioactive iodine-131. CD45 plays a crucial role in the function of hematopoietic cell activation and differentiation. By specifically targeting CD45, a cell surface antigen widely expressed on hematopoietic (myeloid and lymphoid) cells, but not other tissues, Iomab-B can effectively offer target-specific ablation as a conditioning regimen prior to bone marrow transplantation with the potential for improved efficacy and safety / tolerability. And, because CD45 is expressed on both normal and leukemic cells, it can be used to target marrow in both remission and relapsed patients.
The concept of Iomab-B holds significant scientific validity. Above I outlined the need for myeloconditioning / myeloablation prior to BMT; however, I also outlined the risk. Failure to ablate enough cells can result in high relapse rates or increased risk of GvHD, whereas overly intense therapy causes increased risk of treatment-related mortality. Selective radiation of leukemia using radiolabeled monoclonal antibodies against antigens on marrow cells promise to improve results by targeting malignant cells and causing less systemic damage (Matthews DC, et al., 1999).
Iomab-B development is initially focusing on the treatment of elderly patients with refractory / relapsing AML. These are patients that have failed the “7+3” induction phase of chemotherapy and cannot tolerate intensive or even reduced conditioning myeloablation. There is no treatment option for these patients today. These elderly patients fall into the “poor risk” category where salvage chemotherapy offers response rates below 20% and long-term survival rates below 10% (Mangan J., et al., 2011). Many are simply placed on palliative care.
A Phase 1 study conducted in 59 patients with refractory / relapsing AML demonstrated impressive targeted delivery of radioactive isotope with Iomab-B. Results show that 52 (88%) patients receiving Iomab-B had higher estimated absorbed radiation in bone marrow and spleen than in any other organ (Pagel JM, et al., 2006). Specifically, the mean concentration of radioactive isotope in the marrow was 11.3 Gy, 29.7 Gy in the spleen, and 5.25 Gy to the liver in 46 patients receiving the therapeutic dose. Iomab-B biodistribution to the lungs and kidneys was minimal. The estimated 3-year non-relapse mortality and disease-free survival for these patients was 21% and 61%, respectively. Importantly, the initial work with Iomab-B showed an overall incidence of GvHD similar to historic standard conditioning.
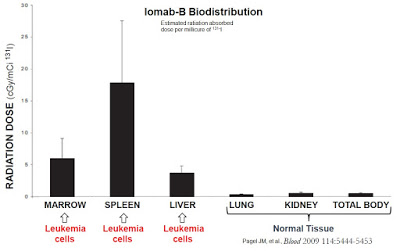
This is important because peers have shown consistently correlation between response rates and whole-body radiation dose. For example, work done by Clift RA, et al., 1990 found a relapse rate of only 12% for AML patients receiving 15.75 Gy TBI compared to a 35% relapse rate for patients receiving only 12 Gy TBI. The author found a similar direct correlation between TBI dose and response rate in patients with chronic leukemia (Clift RA, et al., 1991); however, in both studies the higher TBI exposure was associated with significantly higher treatment-related mortality, such that there was no difference in long-term disease-free survival between the two randomized groups.
Results from the Iomab-B Phase 1/2 study (NCT00008177) were published in Blood in 2009. The trial was sponsored by the Fred Hutchinson Cancer Research Center. The trial was designed to determine the maximum tolerated dose of Iomab-B that can be combined with a standard reduced-intensity conditioning regimen ahead of allogeneic BMT. A total of 58 patients older than 50 years with advanced AML or MDS were treated with Iomab-B and FLU plus 2Gy TBI. Results showed that Iomab-B can be safely combined with RIC (MTD of 24 Gy to the liver), while delivering convincingly strong response. For example, the treatment produced a complete remission in all patients. Seven patients (12%) died of non-relapse causes by day 100. The estimated probability of recurrent malignancy at one year was 40%, and the 1-year survival estimate was 41% (Pagel JM, et al., 2009).
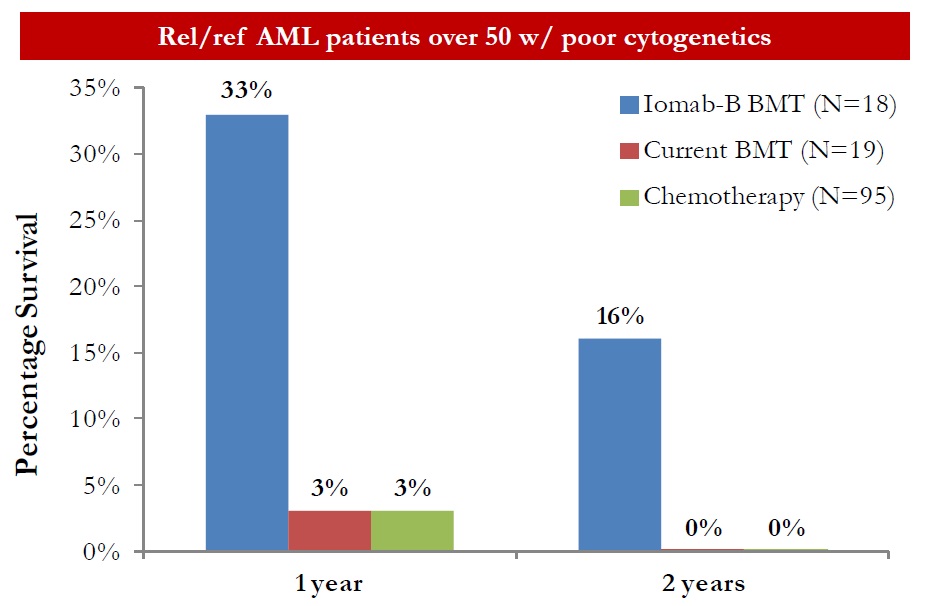
Data from the Phase 1/2 trial was compared to standard conditioning and chemotherapy (outcome analysis compiled by the MD Anderson Cancer Center) and presented by Dr. Hillard M. Lazarus, MD, FACP, Director of Novel Cell Therapy at Case Western Reserve University. In a subset of 18 elderly patients with poor cytogenetics, Iomab-B when added to FLU plus 2Gy TBI resulted in a 1-year overall survival of 33% compared to 3% for standard conditioning and high intensity chemotherapy. After two years, 16% of the Iomab-B cohort was still alive compared to 0% for standard conditioning and chemotherapy (unpublished).
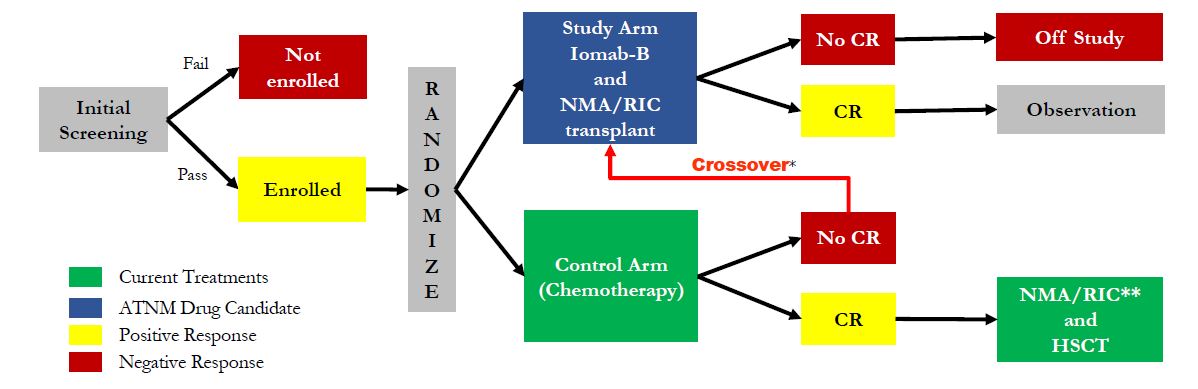
Actinium is planning to initiate a Phase 3 clinical trial with Iomab-B in the U.S. in early 2016. Management is currently preparing to file a U.S. investigational new drug (IND) application before the end of the year. Once cleared, the company should be in position to begin enrollment in the trial quickly thereafter. Management has already held a pre-IND meeting with the agency to discuss the design for the registration study. All signs point to one single study (pending results) being sufficient to gain approval. The Phase 3 trial will likely enroll 150 patients total, all elderly (> 55 years old) with refractory AML. Patients will be randomized between Iomab-B + BMT and salvage chemotherapy. A crossover design has been set up for patients not responding to salvage chemotherapy, which history suggests will be in excess of 80% of the target population. The protocol can be seen below. I suspect the trial will take 12 months to complete, with data in early 2017.
The market opportunity with Iomab-B is significant in my view. There are no currently approved treatment options for elderly patients with refractory AML. This is a patient population of approximately 15,000 individuals in the U.S. and EU each year, less than 20% of which are able to tolerate conditioning regimens that allow them to go onto BMT. I see two potential pathways to meaningful revenues here. The first is in the small subset of patients where the physicians believe reduced intensity conditioning may be tolerated but that Iomab-B may be more effective (~3,000 patients) and the second in which a regimen such as Iomab-B may be more tolerable and allow a physician try to BMT without undue risk of treatment-related mortality (~12,000) patients. Market share gains in both segments seem achievable, and at approximately $60,000 for a course of treatment, Iomab-B targets a $900 million peak opportunity.
Both Phase 1 and Phase 2 trials with Iomab-B have led to effective cures in patients with no options. Once commercialized, I believe it makes sense for Actinium to test Iomab-B earlier in the treatment paradigm for these elderly “high risk” patients, or even move Iomab-B clinical studies into new indications such as myelodysplastic syndrome, acute lymphoblastic leukemia, Hodgkin’s disease, and non-Hodgkin lymphoma. From a mechanistic standpoint, pursuing these follow-on indications holds merit and could open up potentially billions of dollars in additional revenue opportunity for the company or its commercial partner.
The company’s second key clinical-stage asset is Actimab-A, a next-generation monoclonal antibody linked to radioactive actinium-225 (Ac-225). Actimab-A consists of lintuzumab, a humanized monoclonal antibody that targets the myeloid progenitor cell protein CD33. CD33 is a transmembrane receptor leukocyte antigen primarily expressed on cancer cells of myeloid lineage, although it can also be found on some lymphoid cells. Lintuzumab was originally developed by Seattle Genetics with ties back to Memorial Sloan Kettering. Seattle Genetics attempted to develop lintuzumab as a treatment for AML and other myeloproliferative diseases, but a Phase 2b clinical trial failed to demonstrate an increase in overall survival and the program was discounted in 2010 (source).
The concept of Actimab-A holds merit my view. Lintuzumab was proven to be safe and tolerable in the Seattle Genetics clinical work and CD33 is a commercially validated target. Pfizer’s Mylotarg® (gemtuzumab ozogamicin) was an anti-CD33 murine antibody conjugated to the chemotherapeutic agent, calicheamicin hydrazide. The drug demonstrated success in mid-stage clinical trials (Hamann PR, et al., 2001), earning Pfizer accelerated approval from the U.S. FDA for the drug in 2000; however, safety concerns and lackluster post-approval trials ultimately led to the drug being withdrawn from the market in 2010.
Lintuzumab by itself has proven to be ineffective, but linked to the alpha particle–emitting radioactive isotope Ac-225, Actimab-A should see a powerful increase in cancer cell killing effect. Actimab-A is a next-generation product to Actinium’s previous lintuzumab-linked alpha particle emitter, Bismab-A. Bismab-A utilized bismuth-213 and demonstrated positive results in a Phase 1/2 clinical trial – complete response rates in the 30% range, superior to that of Mylotarg® – but the drug was hampered by the short half-life of bismuth-213 (~45 minutes) and lacked commercial viability due to the high cost of goods and complex on-site preparation. Actinium-225 has a half-life of approximately 10 days and should offer significantly improved costs with centralized manufacturing.
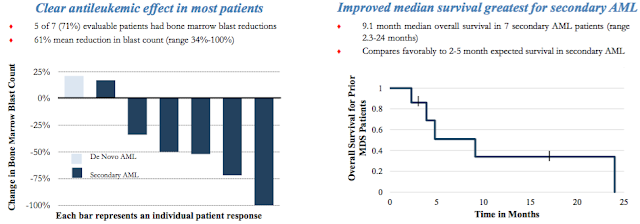
Actimab-A Phase 1 clinical data was conducted in collaboration with the Memorial Sloan Kettering Cancer Center. Results from 18 patients (median age, 64 yrs; range, 45–80 yrs) demonstrated no acute toxicities in doses up to 3-4 microCurie/kg. Importantly, toxicities outside of the target organ (bone marrow) were limited to transient grade 2/3 liver function abnormalities. Follow-up analysis showed no evidence of damage to kidneys due to radiation. Peripheral blood blasts were eliminated in 10 of 16 (63%) evaluable patients who received a full treatment dose. Bone marrow blast reductions of over 33% were seen in 10 of 15 (66%) evaluable patients at 4 weeks, including 3 patients with 5% or fewer blasts.
Actinium Pharma is currently in a multi-center Phase 1/2 clinical trial (NCT01756677) with Actimab-A as a first-line treatment for AML patients over the age of 60. The trial is being conducted at five centers in the U.S., Memorial Sloan Kettering, Fred Hutchinson, Johns Hopkins, Baylor Cancer Center, and the University of Pennsylvania – an impressive group of investigators, no doubt.
The trial is designed to enroll a total of 72 patients in two phases. The goal of the Phase 1 part is to find the highest tolerable dose of Actimab-A that can be given with cytarabine to patients with AML. The goal of the Phase 2 part of this study is to learn if Actimab-A and cytarabine can control AML superior to cytarabine alone. The safety of this drug combination will also be studied. In October 2015, the company announced completion of enrollment in the final cohort of the Phase 1 portion, and later in the month the company announced that data from the Phase 1 portion will be presented in poster form at the 57th American Society of Hematology Annual Meeting being held December 5 – 8, 2015 in Orlando, Florida.
The target market for Actimab-A is meaningful in my view. Approximately two-thirds of the AML population is over the age of 60 years old and half of these patients are not eligible for the current standard of care due to overlapping toxicities with contaminant medications, existing co-morbidities, and/or physician expectations on the patient’s ability to tolerate 7+3 induction. In December 2014, Actimab-A was granted Orphan Drug status by the U.S. FDA (see release). I estimate the patient population in the U.S. is approximately 6,500 individuals. The opportunity in Europe is another 7,500. Assuming a cost of approximately $65,000 per treatment in the U.S. and Japan and $42,500 in the EU (management guidance), the peak opportunity for Actimab-A is easily $750 million. With 33% market share, Actimab-A looks to have sales in the $250 million range.
Conclusion
Acute Myeloid Leukemia is the most common form of leukemia, accounting for roughly one-third of all adult leukemias in the U.S. According to the U.S. National Cancer Institute, there will be estimated 20,800 new cases and 10,500 deaths attributable to AML in the U.S. in 2015. The five year survival rate is only 25.9%. The disease is most common in the elderly, with the average age of onset approximately 67 years old, with the vast majority of patients between the ages of 55 and 75 years old. Many of these patients cannot tolerate the standard of care due to overlapping toxicities with contaminant medications, existing co-morbidities, and/or physician expectations on the patient’s ability to tolerate the medication.
Actimab-A offers an improved efficacy boost to an anti-CD33 monoclonal antibody, lintuzumab, previously demonstrated to be safe in elderly patients with AML through the use of an alpha particle emitting radioisotope, actinium-225. CD33 is a commercially validated target for AML and Actimab-A is a second-generation product to the company’s Bismab-A product candidate that utilized bismuth-213 and demonstrated positive results in a Phase 1/2 clinical trial. A Phase 1/2 clinical trial with Actimab-A is currently ongoing and the company plans to present initial safety and dosing-finding data at the 57th American Society of Hematology Annual Meeting in early December 2015. I see Actimab-A as a $250 million product for the company.
Iomab-B is being developed as a safer and more effective conditioning regimen for patients undergoing hematopoietic stem cells transplant as a potential curative therapy for AML. Iomab-B is a combination monoclonal antibody that targets a lymphocyte common antigen, CD45, and radioactive iodine-131. The drug is designed to offer hematopoietic cell-specific ablation with improved efficacy and safety / tolerability in remission and relapsed AML patients. The concept holds significant scientific merit in my view, as selective radiation of hematopoietic cell using target-specific radiolabeled monoclonal antibodies should be highly effective while causing less systemic damage. I see Iomab-B addressing a significant market opportunity, in AML and beyond, in excess of $1 billion annually.
Actinium Pharmaceuticals exited the third quarter ending September 30, 2015 with $24.8 million in cash and equivalents. The company has two product candidates moving through the pipeline that offer the potential to meaningfully improve treatment outcomes for patients with AML. Research ties and collaborations are in place with well-respected and leading cancer research institutions such as the Fred Hutchinson Cancer Research Center and Memorial Sloan Kettering Cancer Center. The market capitalization of only $101 million (basic) looks to dramatically undervalue these key assets and the long-term potential Actinium presents for investors.


