A team of researchers from Gothenburg University in Sweden presented data from a Phase 4 study on Ceplene® earlier this week at the American Association for Cancer Research (AACR) Annual Meeting in New Orleans, LA. These data could have a profound impact on future use of the drug in elderly patients with Acute Myeloid Leukemia (AML). Ceplene®, whose rights in the U.S. belong to Immune Pharmaceuticals (IMNP), is already approved in Europe and Israel. Immune plans to use these data to attract a development partner for Ceplene®, as the company prepares to advance the drug towards regulatory approval in the U.S.
Quick Background on Ceplene
Ceplene® is a drug Immune acquired in 2013 with the takeover of EpiCept. Until recently, Ceplene® sat on the back-burner at Immune. However, recent data has piqued the interest of management and now it seems as though there is a potential to monetize Ceplene® through a partnership that aims to fund clinical studies designed to gain regulatory approval in the U.S. Ceplene® was approved in the EU in October 2008. EU rights are controlled by Swedish-based Meda Pharmaceuticals. Meda recently refocused its business on respiratory, dermatology, and pain / inflammation products, so the company does not actively promote the product. As such, it is likely that any company interested in the U.S. rights to Ceplene® may also be interested in acquiring the EU rights as well. This presents an interesting in-licensing / out-licensing opportunity for Immune.
Ceplene® (histamine dihydrochloride), in combination with interleukin-2 (IL-2), is believed to work by activating T and Natural Killer (NK) cells into a tumor killing mode. Ceplene® binds to the histamine H2 receptors (H2R) that are predominantly expressed on FAB subtypes with monocytic differentiation. Binding to H2R results in down-regulation of NOX2, which results in decreased production of Reactive Oxygen Species (ROS), and subsequent protection of deleterious immune cells from deactivation and death.
Phase 3 data with Ceplene® used in combination with IL-2 demonstrated an improvement in leukemia-free survival (LFS) in patients with AML. Specifically, complete remission patients in the post-consolidation phase of treatment for AML receiving Ceplene® plus IL-2 achieved a 3-year LFS rate of 40% vs. only 26% for a no treatment control (Brune et al., 2006). Both the European Medicines Agency (EMA) and the U.S. Food and Drug Administration (FDA) have granted Ceplene® orphan drug status for the treatment of AML.
Phase 4 Data At AACR
Earlier this week, at the AACR annual meeting, researchers out of Sweden presented data on the Phase 4 MISSION trial, designed to assess the immunomodulatory properties of Ceplene® plus IL-2, and to correlate potential biomarkers with clinical outcome. In the Phase 4 international study, investigators found a reduction in the frequency of CD8+ T effector memory (TEM) cells during the first cycle of Ceplene® + IL-2 prognosticated leukemia-free survival (LFS) (HR 0.25, P=0.001) and overall survival (OS) (HR 0.24, P=0.009). Similarly, induction of T effector cells (TEFF) during the first cycle also impacted favorably on patient outcome (P=0.048 for OS).
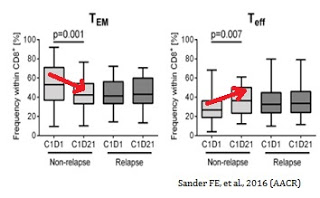
The figure below, taken from the AACR Poster (Sander FE, et al., 2016), shows that a reduction in TEM cells during the first cycle (21 days) of Ceplene® + IL-2 was evident and statistically significant in non-relapsing AML patients (n=18), but not evident in relapsing patients (n=26). Data also show an increase in the frequency of TEFF cells during the first cycle of Ceplene® + IL-2 for non-relapsing AML patients (n=18) but not in relapsing patients (n=26).
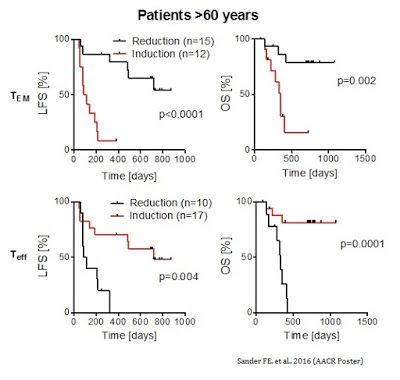
As further evidence by the MISSION data, a reduction in TEM cells during the first cycle of Ceplene® + IL-2 led to an improvement in both LFS and OS in all patients. The results were particularly prominent in patients over the age of 60. Similarly, an induction of TEFF cells during the first cycle of Ceplene® + IL-2 led to an improvement in both LFS and OS in all patients, again, with particular prominence in patients over the age of 60.
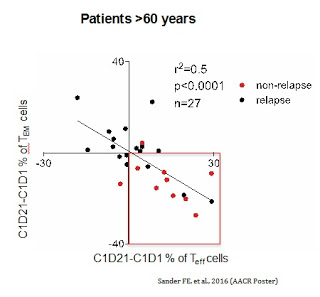
The concomitant increase in TEFF cells and a decrease in TEM cells was the strongest predictor of complete response and improvement in LFS (HR 0.19, P=0.001) and OS (HR 0.13, P=0.008) in older patients (> 60 years) with high risk relapsed AML.
Thus, the altered distribution of cytotoxic T cells during the first cycle (21 days) treatment with Ceplene® + IL-2 significantly prognosticated LFS and OS, especially within the group of elderly patients. These data provide a potential personalized approach for treating therapy-resistant older AML patient populations, in addition to the selection of the population most likely to benefit by FAB subtype. Keeping AML patients in remission post-consolidation is a major challenge, so Immune plans to explore further the combination of Ceplene®+ IL-2 formulations in 2016.
A Place For Ceplene In The Market
Recent breakthroughs in immuno-oncology (e.g. CAR-T, checkpoint inhibitors, liposomal 7+3, etc…) have significantly changed the potential treatment paradigm for AML, so the future for a drug like Ceplene® is likely in combination with newer advanced therapeutics. In this regard, the company is seeking a new patent for the combination of Ceplene® and checkpoint inhibitors. The new invention provides methods of treating cancer by administration of Ceplene® in combination with immune checkpoint inhibitors, as well as methods for predicting the efficacy of Ceplene® and IL-2 therapy in patients with AML.
The biomarker data presented at AACR suggests an interesting potential personalized approach to the selection of patients who are most likely to benefit from Ceplene® + IL-2 maintenance therapy in AML. AML is a horrible disease with little in terms of breakthrough treatments approved over the past decade. Recently, Celator Pharmaceuticals (CPXX) posted some positive Phase 3 top-line results with Vyxeos® as an induction therapy. Celator shares are up over 1,000% from that news. Ceplene® + IL-2 as a maintenance therapy looks to add to the recent success in treating AML patients.
For example, oncologists can analyze T cell phenotypic expression before and after the first cycle of Ceplene® + IL-2 looking for an increase in TEFF cells and a reduction in TEM cells. With confirmed response, patients can be kept on Ceplene® + IL-2 therapy used along with immunotherapy with checkpoint inhibitors or other personalized approaches. Lack of predictive response can also be identified if the T cell expression does not correlate to the ideal altered distribution, and those patients can be moved to alternative medications.
Immunes I/O Strategy Intrigues
As noted in previous articles, Immune is expecting to enter into a transaction in the coming months to create an immune-oncology subsidiary separate from the company’s focus on inflammation and dermatology with bertilimumab. A transaction that brings about significant funding to the company should help return investor interest to the story. I see a lot of potential for Immune’s drug candidates in inflammation, dermatology, and immune-oncology, but funding for these programs and remains a key area of investor concern. New data on drugs like Ceplene® and advancing programs wth NanomAbs® and bi-specific antibodies has the potential to attract much-needed capital and turn around the shares of Immune Pharmaceuticals.


