The psychiatric community seems on the verge of a medical breakthrough to treat depression, or more specifically, major depression that is resistant to traditional antidepressants like Prozac®, Paxil®, and Zoloft®. The focus is on the NMDA-receptor, and it stems from a recent discovery that ketamine, the psychiatric “party-drug” of the 70’s, is both highly effective and rapidly active in reversing major depression in treatment-resistant patients.
VistaGen Therapeutics (NASDAQ: VTGN) is developing AV-101, a glycine B (GlyB) receptor antagonist that negatively modulates the N-Methyl-D-aspartic acid (NMDA) receptor and may induce synaptogenesis. It’s a fundamentally different pathway from standard antidepressants like Prozac® or Lexapro®, and similar to the glutamatergic AMPA-dependent pathway of ketamine; but, without the potential negative side effects of NMDA ion channel blocking.
I wrote an article in June on VistaGen for investors (LINK) that goes over the background on NMDA, the science of AV-101, and why the drug might be a blockbuster for major depressive disorder (MDD). In July, the company appointed Dr. Mark A. Smith, M.D, Ph.D. Chief Medical Officer. Dr. Smith is a prominent research psychiatrist with more than 20 years of experience in basic research and CNS drug development from the lab bench through clinical proof of concept studies. I scheduled time with Dr. Smith for a talk about the depression market, AV-101, and where VistaGen is heading over the next few quarters.
Jason Napodano: Mark, thank you for taking the time to speak with me today! Let’s start out with why you joined VistaGen. You were working at Teva Pharmaceuticals as the Clinical Lead for Neuropsychiatry. Teva is obviously making a strong push into CNS. They bought Auspex a year ago, NuPathe two years ago, and we can even go back to the acquisition of Cephalon as evidence of their push into this area. It’s clear Teva is trying to become a CNS leader; but, you left them to come to VistaGen. Why?
Mark A. Smith: It is my pleasure, Jason. Yes, Teva has been and continues to be focused on CNS disorders, so it was not a trivial decision to leave my friends and colleagues there. However, during a 13-year stint at my previous employer, AstraZeneca, I had the privilege to work on another NMDA antagonist known as lanicemine or AZD6765. It had the potential to be a “kinder and gentler” ketamine, and although AZD6765 never made it to market, that experience inspired me to continue to seek out novel glutamatergic drugs that might have the antidepressant efficacy of ketamine without the side effects. VistaGen may have such a drug in AV-101. You don’t often get a second chance in life, but when you do, you grab it!
JN: You’ve obviously got tremendous experience in developing drugs to treat CNS disorders, including depression and bipolar disorder. Let’s talk about IV ketamine and its use today for treatment-resistant depression. I’ve read a lot about this topic and just about everything says it is highly effective. I’ve even had a few patients reach out to me and say it has been a lifesaver, literally. Can you give us a sense of how common use of ketamine is to battle TRD and what are some of the issues that patients on ketamine might face?
MS: The serendipitous finding that ketamine is a rapid-acting antidepressant for treatment-resistant depression is undoubtedly the most important discovery in neuropsychiatry in at least the past 20 years. Although not FDA-approved for treatment of depression, a number of very reputable academic medical centers have set up ketamine clinics. In addition, non-academic ketamine clinics are popping up throughout the U.S. The fact that depression patients are spending nearly $5,000, out of pocket, per four-infusion course of therapy for ketamine treatment shows how desperate they are when they run out of treatment options. I agree that ketamine can be a lifesaver. However, ketamine has quite a bit of baggage, including cardiovascular effects, the potential to cause transient psychosis, not to mention that it is a scheduled drug with potential for abuse. For those reasons and a few others, it is often administered by an anesthesiologist in a clinical setting similar to where electroconvulsive therapy (ECT) is delivered.
JN: The term “treatment-resistant major depression” is a little nebulous. Can you help us understand what defines the term treatment resistant? How many traditional SSRI or SNRI drugs does someone need to fail before a physician might consider them to be resistant, and try something like ketamine?
MS: TRD usually refers to patients who have failed to respond to two different standard antidepressants. By fail, I mean they have not had a 50% reduction in their symptoms after a 6-8 week course of treatment (the time it takes for most SSRIs and SNRIs to work). In practice, many of these patients have failed on SSRIs, SNRIs, atypical antipsychotics and even ECT before trying ketamine.
JN: I would assume there are payor implications of that definition? Is ketamine reimbursed?
MS: Ketamine infusions are expensive and are not generally reimbursed yet prior to its approval by the FDA as an adjunct antidepressant for TRD. However, I have heard that a few insurance companies are already beginning to partially reimburse for it because they recognize that untreated TRD is not only a potentially life-threatening disorder but also poses a tremendous burden on the mental health care system if the patient must be hospitalized or receive ECT etc.
JN: The CDC and NIMH peg the number of depressed Americans at around 18 million. If we assume 50% of these patients will experience a major depressive episode over a 12 months window and then use your definition above for TRD patients, then we are looking at a target market in the U.S. of potentially over 3 million patients (see image below). Within that population, what percent do you think ketamine, or more specifically, drugs that target the NMDA-receptor, can help?
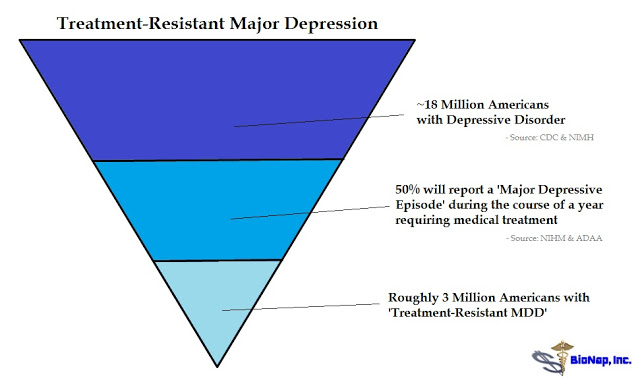 MS: In the context, we see it as larger, but based on the experience so far, it appears that NMDA drugs may be able to help at least 50-60% of TRD patients. That is obviously a substantial number of patients who otherwise would continue suffering whether it is 3 million or even larger.
MS: In the context, we see it as larger, but based on the experience so far, it appears that NMDA drugs may be able to help at least 50-60% of TRD patients. That is obviously a substantial number of patients who otherwise would continue suffering whether it is 3 million or even larger.
JN: I read that ketamine can snap even the most severely depressed patients back to a healthy state in as little as one dose. Hospitals and mental institutions use single doses of ketamine to prevent suicide in high-risk patients. How long does that effect last?
MS: As you know, suicide rates have increased alarmingly in the U.S. over the last 15 years. And yes, the NIMH experience has been that a single ketamine infusion relieves suicidal ideation very rapidly above and beyond its effects on anxiety and depression. Janssen recently showed that intranasal esketamine also rapidly relieved suicidal ideation in a placebo-controlled study. Several other open-label studies in and out of the emergency room have seen similar results. But the question you rightly ask is how long does the effect last? Although it varies from patient to patient, a single dose of ketamine relieves depression and suicidal thoughts for 1-2 weeks, perhaps occasionally longer, but it’s certainly not a cure.
JN: From a patient or payor perspective, a one pill solution is phenomenal; but, from a biopharma company standpoint, it’s difficult to make money with that business model. I assume you see a business model for NMDA-receptor targeting drugs or else you wouldn’t be at VistaGen. Can you help us understand how you see these drugs being used?
MS: Well I don’t think there really is a one pill solution. I believe that ketamine will be used to “jump start” TRD patients out of their acute depression. However, the problem then will be how to sustain this antidepressant effect on a long-term basis. Most patients will relapse if not treated chronically. A recent study by Dawn Ionescu at Harvard found that the anti-suicidal effect could be sustained in some patients by repeated doses of IV ketamine (see: J Clin Psychiatry, 2016).
So I think it is clear that major depression is a chronic disease that requires chronic treatments. Some patients may opt to come into the clinic periodically for “booster shots” of ketamine similar to “maintenance ECT”. But this is very burdensome for the patient. A much better solution would be if we had a safe and effective NMDA drug that could be administered at home by the patients themselves. Ideally, that drug should be taken orally.
JN: Let’s talk about AV-101. Above you told us why VistaGen, and obviously AV-101 key driver at this point, but what is it about AV-101 that gets you excited? Because I see in your background you’ve been involved in filing over 20 investigational new drug (IND) applications in CNS. So what makes AV-101 special?
MS: Well as I said, AV-101 has the potential to be a “kinder and gentler” ketamine that is orally bioavailable and very safe, all of which makes it ideally suited to be given chronically to patients suffering from TRD. The recent publication by Zanos et al, 2015, from the University of Maryland proved that AV-101 shows consistent antidepressant effects in a number of rodent models. These results were equal to those seen with ketamine and superior to established antidepressants like fluoxetine. Other laboratories have seen similar results with 7-chloro kynurenine (the active ingredient in AV-101). These preclinical antidepressant properties of AV-101 are as robust and consistent as I have seen, and they practically demand that AV-101 be tested in depressed patients.
I have worked on a number of first-in-class drugs. Many were stopped on account of safety or toxicity reasons. I am already confident that AV-101 will be proven safe and well tolerated based on the Phase 1 studies in normal volunteers so far. Now, we just need to prove it is as efficacious in patients as it is in rodents.
JN: I also see in your background you’ve spent some time as Senior Staff Fellow at the National Institute of Mental Health (NIMH). Dr. Carlos Zarate, MD at NIMH, one of the nation’s foremost experts in depression is the principal investigator of an ongoing NIH-funded Phase 2a study (NCT02484456) with AV-101. Before we get into the details of that program, can you tell us a little about the NIMH, in terms of what the institute looks for when it decides what drugs to sponsor? What trials to run? Because they can spend money wherever they want; literally there are hundreds of molecules to choose from. It’s confidence-inspiring to see the NIMH and Dr. Zarate involved with AV-101.
MS: Actually I first learned of AV-101 during a conversation with Carlos. He began testing AV-101 prior to my joining VistaGen. And yes, he does have his pick of many compounds to test in his unique setting at the NIH Clinical Center. First and foremost, Carlos is a scientist and picks compounds based on their preclinical and clinical evidence that they have antidepressant properties. Obviously, the preclinical evidence on AV-101 from Zanos et al was persuasive. Although the majority of his studies have involved NMDA compounds, this has more to do with the success of this mechanism for depression than any bias on his part.
Coincidentally, and quite separate from VistaGen, I serve on the NIMH Neuropsychopharmacology Task Force to assist the NIMH investigators to prioritize compounds, and we consider a wide variety of mechanisms. But more often than not, we are discussing glutamatergic compounds because of the breakthrough results with ketamine.
JN: Obviously a very important study for VistaGen. Give us a run-down of the ongoing NIH-funded AV-101 Phase 2a study and what you’re expecting to learn from that read-out during the first half of 2017.
MS: I have had the pleasure to work with Carlos and his excellent staff on two previous AstraZeneca compounds for depression and anxiety, and so I am very pleased that he took an interest in AV-101. The inpatient unit at NIMH is almost unique in its ability to observe patients in a controlled setting for weeks or even months. The careful and close observation makes for a low placebo response that can bring out an antidepressant signal using a relatively small number of TRD patients.
The NIMH group is testing AV-101 as monotherapy for depression for 14 consecutive days in a placebo-controlled cross-over design in about 25 TRD inpatients. This is similar to the many cross-over studies they have conducted with IV ketamine. So I expect that they will be able to determine if daily administration of AV-101 also demonstrates an antidepressant effect in this treatment-resistant group. I am hopeful the side effect profile will be placebo-like. The NIMH will also do some imaging to detect whether AV-101 might affect glutamate levels in the brain similar to what ketamine does. This would add to our understanding of exactly how AV-101 is working in the brain. This will be the first proof of principle in man that AV-101 has antidepressant effects in humans, so we are obviously excited.
JN: VistaGen is planning to initiate a separate, larger Phase 2b study in the coming months. Can you talk a little about that program, and specifically highlight what I think is an interesting protocol design?
MS: Sure, the main differences between the NIMH Phase 2a study and the proposed Phase 2b study are that the proposed Phase 2b will be conducted in outpatients, and AV-101 will be administered on top of background antidepressant therapy that is not adequately treating these patients. There are many questions that need to be answered. One is dose. In the proposed study, we will test both a high dose 1440 mg and a lower dose of 720 mg which should produce blood levels of AV-101 in the range of what was found necessary in the rodent models. We plan to utilize a regimen of two times per week similar to the regimen being used for ketamine.
As to the design, we expect to use a sequential parallel comparison design (SPCD) made popular by Dr. Maurizio Fava and colleagues at Harvard and aimed at weeding out placebo responders (see image below). Frankly, in this day and age, I would not conduct a traditional parallel design to test a novel antidepressant. Like many others, I have had the frustrating experience of being unable to show that a proven drug like an SSRI separates from placebo using a parallel design. Instead, we will utilize the SPCD to identify placebo non-responders and then re-randomize them to either continue on placebo or switch to AV-101. In this way, we should be able to detect an antidepressant response.
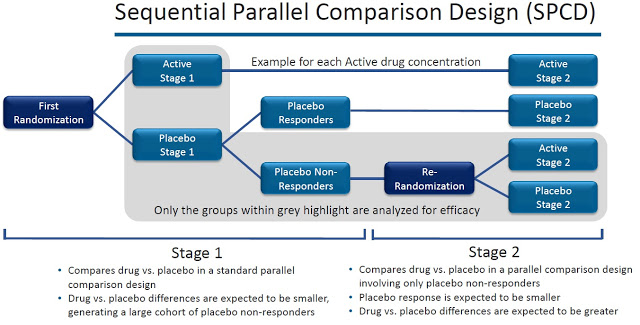
As to the players set to execute the study, I’d be hard-pressed to find a more accomplished team, one with more relevant real-time clinical development and regulatory experience, than the team VistaGen has on board, and had on board well before I joined. Drs. Zarate, Fava, Sanacora, and Mathew are among the best in the world at what they do and have been doing to help care for depression patients for decades. Their involvement with VistaGen and AV-101, the collective expertise they bring to bear on our trial planning and execution, is a tremendous benefit to VistaGen, and was a major factor in my decision to leave Teva and join the company at this key pivot point.
In addition to the clinical experts, Dr. Tom Laughren, former director of the FDA’s Division of Psychiatry, is also on board providing us with valuable regulatory insight and guidance on matters associated with our Phase 2 program. Finally, we are working with elite CROs – PPD, Cato, and CTNI at Mass General – all of whom are highly experienced in CNS drug development and give me firm confidence that they will take good care of our resources and enable us to execute our Phase 2b program efficiently and effectively.
JN: Mark, I wrote an article last month about NMDA-targeting for depression (LINK). I highlight a lot of potential players, including some big boys like J&J and Allergan. That’s a concern for some investors. I know this is a big market that can sustain multiple players. For example, I noted in that article that big pharma companies have a history of promoting multiple drugs in this space, Zoloft®, Effexor®, and Pristiq® all that Pfizer, Paxil® and Wellbutrin® both at Glaxo, Celexa® and Lexapro® both at Forest Labs, Prozac® and Cymbalta® both at Eli Lilly. Can a little guy like VistaGen compete in this field?
MS: Great article by the way! Early drug development is my forte, and together with the high-quality team we have on the field, yes, I think we can compete with anybody, at any level. Plus, we have gained tremendously from the experience of those who have gone before us. With a potentially game-changing drug candidate such as AV-101, especially a new, safe, oral alternative in massive global CNS markets for depression and the other indications for which we believe AV-101 has commercial potential, it is just simply good business practice to socialize that opportunity with a range of potential strategic partners, even at this stage, and ultimately align with one having well-established, global commercial reach in the CNS sector, or possibly one with an actual and obvious financial commitment to developing that core competency in the near term, such as seems the case with several large cap biotechs with Phase 3 or newly approved CNS assets. As we have seen in at least one other case recently, if we hit our targets, we expect robust opportunities to deliver extraordinary value to CNS patients and our stockholders.
JN: Last year Allergan acquired privately-held Naurex for $572 million in cash upfront and over $1 billion in potential cash flows downstream. Naurex was, and now Allergan is, developing a similar drug to AV-101 called rapastinel. Can you give us a sense of how oral AV-101 compares and contrasts to IV rapastinel?
MS: Yes, that was quite a deal – that was the deal to which I was alluding a minute ago. I applaud their efforts and all the nice translational work done by Joe Moskal’s group at Northwestern. Rapastinel is a four amino acid peptide that, as you noted, must be given intravenously, whereas AV-101 is small molecule prodrug that is given orally. Rapastinel’s plasma half-life is measured in minutes (about 10 minutes), whereas AV-101 has a half-life in plasma of about 3 hours. Both rapastinel and the active ingredient in AV-101, which is 7-chlorokynurenine, have been shown to bind to the glycine modulatory site on the NMDA receptor, so both seem to down-regulate NMDAR activity rather than blocking the ion channel of the NMDAR as does ketamine.
We believe that modulatory, rather than ketamine-like channel-blocking, activity of AV-101 and rapastinel accounts for the excellent safety profile of the drug candidates. There is some evidence, actually published previously by the Naurex team, that AV-101’s active metabolite may have about a 3x more potent binding affinity to the glycine binding site of the NMDA-receptor than IV rapastinel. Regardless, IV rapastinel has clinical data to support its utility as a new generation adjunct antidepressant, and it will be interesting to see if Allergan’s Phase 3 studies confirm the earlier results from Naurex. It will also be interesting to see if their follow-up compounds, such as NRX-1074, are really efficacious when given orally.
JN: Similar question, but this time with J&J’s esketamine.
MS: J&J is currently undertaking a massive Phase 3 program to prove that intranasal esketamine is an adjunct antidepressant for use in patients with TRD. I believe they will be successful. However, once approved, the big questions are in what setting intranasal esketamine will be delivered and how often must it be administered as maintenance therapy. If patients must return regularly to the doctor’s office or an ECT suite at the local hospital, this may be a deterrent to many patients, although there is an important advantage to those averse to needles and IV infusions. Unfortunately, efficacy and side effects are both correlated to dose, and thus it is difficult to find an efficacious dose free of psychosis etc. Moreover, the intranasal route is the preferred route of abuse of ketamine or “Special K”. Still, I think ketamine and the S-enantiomer will find a lot of users because of its rapid and robust antidepressant effects. The robust current private-pay market activity for IV ketamine therapy, nearly $5k per course of infusions, works into my thinking on that potential outcome.
JN: Big pharma also seems to like to take one drug and develop it for multiple indications. Drugs like Paxil®, Cymbalta®, and Abilify® are perfect examples. VistaGen recently raised $10 million in May 2016. As CMO, that’s got to be exciting. Where do you think AV-101 can go beyond MDD? Is there’s Cymbalta® or Abilify®-type potential here?
MS: Yes, absolutely! There are a number of possibilities, and that was a key factor in my decision to join VistaGen and quarterback development of AV-101. As has been well-publicized over the past several years, ketamine has shown efficacy in a number of indications such as bipolar depression, suicidal ideation associated with depression, obsessive compulsive disorder and of course pain. AV-101 might also have efficacy in these disorders and symptoms, as well as certain neurodegenerative diseases. If AV-101 is as safe as we believe it is and is as unlikely to be abused as we expect, then it could be explored in many other CNS indications, such PTSD and anxiety disorders.
We have seen the effects of NMDA antagonists to relieve anxiety, but a NMDA drug would have to be quite well tolerated and safe resulting in a good risk/benefit ratio to be justified in the treatment of anxiety disorders. AV-101 should have a good risk/benefit ratio which would allow its use even in the elderly where oral ketamine is being used off-label sometimes in palliative care. Finally, NMDA antagonists and glycine antagonists like 7-chlorokynurenine were originally thought to have neuroprotective properties. It may be worthwhile to revisit this in traumatic brain injury or Huntington’s disease with a well-tolerated drug like AV-101. Having successfully completed two Phase 1 safety studies, we now have many opportunities to leverage our Phase 2 IND to explore other indications. Our core focus will remain squarely on MDD, but we will continue to assess other clinical development and commercial opportunities on an ongoing basis.
JN: Mark, I appreciate your time today. Is there anything else you’d like to note about VistaGen or AV-101?
MS: Just that AV-101 has the potential to be the first oral NMDA agent to treat depression. I believe that IV ketamine with its rapid antidepressant effects was the big breakthrough that will revolutionize the treatment of acute depression. However, the challenge going forward will be how to sustain that antidepressant effect for months and years with a drug that is safe and easy to administer. We believe AV-101 has the potential to be that drug.
Conclusion
Depression is a debilitating disease that affects approximately 18 million Americans. Roughly three million of these individuals has major depression that is resistant to conventional antidepressants. Abnormalities of the NMDA receptor contribute to imbalances in glutamatergic neurotransmission that may lead to depressive pathology. Antagonism of the NMDA receptor by ketamine has been shown in both preclinical and clinical models to reverse depressive states rapidly and resolve suicidal ideation after only a single dose.
Several pharmaceutical companies are developing drugs that target the NMDA receptor, including behemoths Johnson & Johnson and Allergan. Micro-cap VistaGen Therapeutics is developing AV-101, an oral byproduct of 7-Cl-KYNA, a full antagonist of the NRI (GlyB) subunit of the NMDA receptor. Investigators at the NIMH are currently studying AV-101 in a Phase 2a clinical trial. The company plans to move AV-101 into a Phase 2b clinical trial in the next few months. I believe if successfully commercialized, AV-101 has blockbuster sales potential.
Post Phase 2 assets for depression are worth a lot of money. I cite three examples. As noted above, Allergan (NYSE: AGN) acquired privately-held Naurex for $572 million in upfront cash and the potential for over $1 billion in milestones in July 2015. In May 2016, Minerva Neurosciences (NASDAQ: NERV) reported positive results from a Phase 2a study with MIN-117, a serotonin and dopamine transporter, in patients with MDD. Minerva added over $360 million in market value shortly after the news. Most recently, Sage Therapeutics (NASDAQ: SAGE) added over $500 million in market value in July 2016 when the company reported positive Phase 2 data with SAGE-547 in only 21 subjects with postpartum depression.
The market seems pretty clear on the valuation inflection potential for VistaGen. Quite simply, report positive Phase 2 data in depression and the company is worth between $400 to $500 million in value. VistaGen’s current market capitalization is only $25 million. The company recently secured $10 million in cash through a public offering in May 2016, so I’d say the stock has all the makings of a potential big winner for investors.
———-
This article was written by Jason Napodano, CFA of BioNap, Inc. Subsequent to the publication of this article, Jason has established a position in VTGN.
Investors should be aware that certain statements contained in this article may be “forward-looking statements” within the meaning of the Securities Litigation Reform Act of 1995. They are generally identified by words such as “believes,” “may,” “expects,” “anticipates,” “should” and similar expressions. Readers should not place undue reliance on such forward-looking statements, which are based upon the company’s beliefs and assumptions.
Investors should understand, the company’s actual results could differ materially due to risk factors and other items described in more detail in the “Risk Factors” section of the company’s Annual Reports and MD&A filed with the United States Securities and Exchange Commission (SEC). Subsequent events and developments may cause these forward-looking statements to change. BioNap specifically disclaims any obligation or intention to update or revise these forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
Please see additional information on our Disclaimer.


