On Tuesday, September 8, 2015, I attended the Immune Pharmaceuticals (IMNP) R&D Day at the company’s U.S. headquarters at the Alexandria Center for Life Science, in New York City. The majority of the event was spent talking about Immune’s lead pipeline candidate, bertilimumab, for the treatment of immune-inflammatory diseases of the skin and gastrointestinal tract. For the purpose of this article, I look at two potential indications for bertilimumab, bullous pemphigoid (BP) and atopic dermatitis (AD), discuss the rationale behind why and how Immune believes their drug works, and what the respective market opportunities might be for the company upon successful development. Future articles will touch on the potential use of bertilimumab for ulcerative colitis, Crohn’s disease, and non-alcoholic fatty liver disease and non-alcoholic steatohepatitis (NASH).
Background on Bullous Pemphigoid and Role of Eotaxin-1
Bullous Pemphigoid is a chronic autoimmune skin disease that involves the formation of blisters, known as bullae, at the space between the epidermis and dermis layers of the skin. The disease may be acute, but typically will wax and wane over a period of several years. The primary affected regions include the inner thighs and upper arms, but the trunk and extremities are also frequently involved. The disease typically affects individuals over the age of 65 years old, with an estimated 65,000 afflicted patients in the U.S. and European “Big 5” (Korman, 1998). It has been designated as an orphan disease by both in both regions.
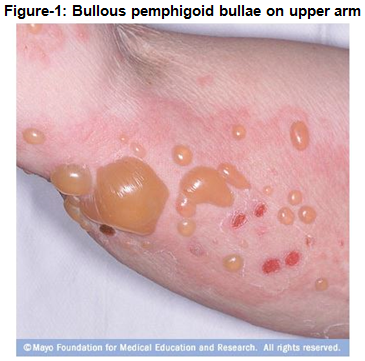
Bullous pemphigoid is a chronic inflammatory disease. If untreated, the disease can persist for months or years, with periods of spontaneous remissions and exacerbations. In most patients who are treated, bullous pemphigoid remits within five years or onset. Patients with aggressive or widespread disease, those requiring high doses of corticosteroids and immunosuppressive agents, and those with underlying medical problems have a nearly two-fold increased morbidity and risk of death (Langan et al., 2008). And, because the average age at onset of bullous pemphigoid is about 65 years, patients with bullous pemphigoid frequently have other comorbid conditions that are common in elderly persons, thus making them more vulnerable to the adverse effects of corticosteroids and immunosuppressive agents (Medscape).
The disease may be fatal, particularly in patients who are debilitated. The primary causes of death are infection with sepsis and adverse events associated with treatment. Patients receiving high-dose corticosteroids and immunosuppressants are at risk for several adverse events (AES) including peptic ulcer disease, GI bleeds, glaucoma, osteoporosis, hypopituitary-pituitary-adrenal (HPA) axis suppression, psychological effects, agranulocytosis, and diabetes. A Swiss prospective study confirmed a high-case fatality rate, with three-fold increased one year mortality compared with the expected mortality rate for age-adjusted and sex-adjusted general population (Cortes et al., 2011).
Onset of the disease is largely idiopathic, but is associated with other nonbullous chronic inflammatory skin diseases such as pruritus, lichen planus, and psoriasis (Bakker CV et al., 2013). BP has been reported to be precipitated by ultraviolet irradiation, x-ray therapy, and exposure to some drugs, including furosemide, ibuprofen, and other nonsteroidal anti-inflammatory agents, captopril, penicillin and other antibiotics.
Clinical manifestations are widespread tense blisters/bullae and urticarial lesions associated with moderate to severe pruritus. The bullae may eventually erupt, which may cause pain and increase risk of infection, but generally the lesions heal without scarring or milia formation. Blistering on the palms and the soles of the feet can severely interfere with patients’ daily functions. Other symptoms include blisters in the mouth, which may cause dysphagia, and redness and soreness of the eyes.
The bullae are formed by an immune reaction, initiated by the formation of IgG autoantibodies targeting Dystonin, also called Bullous Pemphigoid Antigen 1 (BPAG1) or AgBP230 (Iwasaki et al., 1995) and/or type XVII collagen, also called Bullous Pemphigoid Antigen 2 (AGBP2) or AgBP180 (Xu et al., 2000). The binding of IgG autoantibodies at the basement membrane activates complement and inflammatory mediators that lead to a cascade of immuno-modulators resulting in a variable surge of immune cells, including neutrophils, lymphocytes and eosinophils to the affected area. These inflammatory cells are postulated to release proteases, which degrade hemidesmosomal proteins and lead to blister formation.
Eosinophils are characteristically present in human patients’ blisters as demonstrated by histopathologic analysis, although their presence is not an absolute diagnostic criterion. Eotaxin, an eosinophil-selective chemokine, is strongly expressed in the basal layer of the epidermis of lesional bullous pemphigoid skin and parallels the accumulation of eosinophils in the skin basement membrane zone area. It may play a role in the recruitment of eosinophils to the skin basement membrane area (Frezzolini et al., 2004).

Eotaxin-1 is a master regulator of the immune cellular network by inducing key immune cytokine responses. A study published in the European Journal of Dermatology found an increased level of serum eotaxin-1 in BP patients compared to patients with pemphigus vulgaris (PV), another skin blistering disorder, and healthy controls (Frezzolini et al., 2002). In addition, the authors found that eotaxin-1 is correlated with disease severity, with the level of eotaxin-1 in blister fluids found to be approximately 10-fold higher than in corresponding sera and when compared to blister fluid obtained from suction blisters from healthy volunteers.

Results published in the British Journal of Dermatology also showed elevated levels of eotaxin-1 in blister fluid from BP patients. Quite interestingly, level of the eotaxin-1 was strongly associated with dermal infiltrating eosinophils in BP patients, and immunohistochemistry analysis showed that eotaxin was strongly over-expressed in epidermal keratinocytes around BP (Wakugawa et al., 2000, ) but not in healthy controls (Shrikhande et al., 2000). Data published in Clinical and Experimental Immunology examined the level of eotaxin-1 in BP patients stratified by disease severity. The authors found that the highest levels of eotaxin-1 were in patients suffering from the most severe disease, suggesting a correlation between the level of eotaxin-1 over-expression and disease severity (Günther et al., 2011).
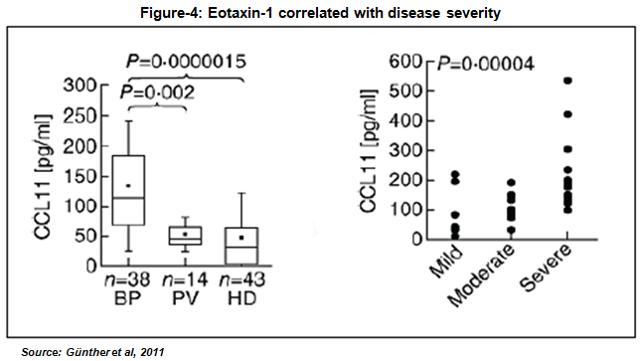
Based on available peer-reviewed literature, it can be concluded that eotaxin-1 is an important mediator of eosinophil transmigration into tissue and its over-expression is highly correlated with manifestation of bullous pemphigoid. Eosinophils co-localized with basal keratinocytes expressing higher eotaxin-1 and CCR3 – CCR is known to be expressed by hematopoietic cells only but a few publications suggest CCR3 to be expressed by non hematopoietic cells as well such as keratinocytes (Petering H et al., 2001) as well as fibroblasts – the main receptor in eosinophils with high affinity for eotaxin-1, in skin lesions and bullae of BP patients. Immune Pharmaceuticals believes that these data indicate eotaxin-1 is involved in the dermal eosinophilia and the pathogenesis of BP, thus making eotaxin-1 a valid therapeutic target for treatment of BP disease.
Current Treatment Options for Patients with BP
There is no cure for BP; thus, treatment options are utilized to reduce the size of blisters and provide symptom relief, which includes inflammation and itching. The most commonly used medications are class-1 anti-inflammatory agents (e.g., corticosteroids, tetracyclines, and clobetasol) and are generally sufficient to reduce flare-ups and safe for short-term use. A study done in Europe provided evidence that strong topical corticosteroid treatment may be as effective as systemic oral corticosteroids in patients with BP while avoiding systemic adverse effects from systemic use (Joly et al., 2002). However, long-term use of high-dose corticosteroids results in significant side effects, including weight gain, glaucoma, diabetes, high blood pressure, hormone imbalances, osteoporosis, and edema and thus is not suitable for patients with severe or recurrent BP (Mayo Clinic).
For more severe cases, systemic steroids such as prednisone along with immunosuppressives (e.g., azathioprine, methotrexate, mycophenolate mofetil, and cyclophosphamide) may be needed to control the disease. Antibiotics such as tetracycline or erythromycin may also control the disease and prevent infection (Olivry et al., 2000). In patients who cannot use corticosteroids, an anti-CD20 antibody (rituximab), which is relatively specific in targeting the antibody-producing B cells, has been found to be effective (Olivry et al., 2000). What seems clear, however, is that new treatment options for patients with severe or recurrent disease are necessary. As noted above, patients with severe disease refractory to anti-inflammatory or immunosuppressive drugs have a three-fold increased risk of mortality. This is a clear unmet need that needs to be addressed.
Background on Bertilimumab
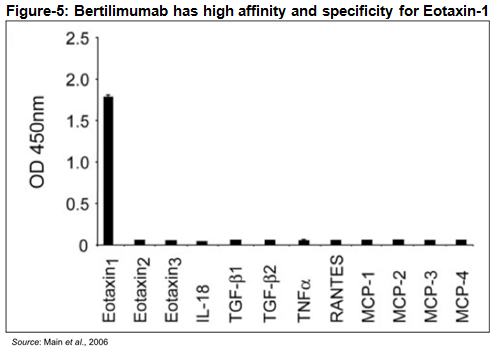
Bertilimumab is a first-in-class fully human IgG4 monoclonal antibody with high target affinity against eotaxin-1. The antibody was originally discovered by Cambridge Antibody Technology (CAT) using is fully human phage display library. Bertilimumab, originally called CAT-213, binds to the aforementioned eotaxin-1 with very high affinity (~80 pM) and specificity. For example, ELISA analysis conducted using a series of human cytokines and chemokines, including the functionally related eotaxin-2 and eotaxin-3, demonstrates the high affinity and specificity of the antibody for eotaxin-1. This high degree of specificity and affinity should decrease any off-target effects or toxicities due to cross-reactivity with other antigens.
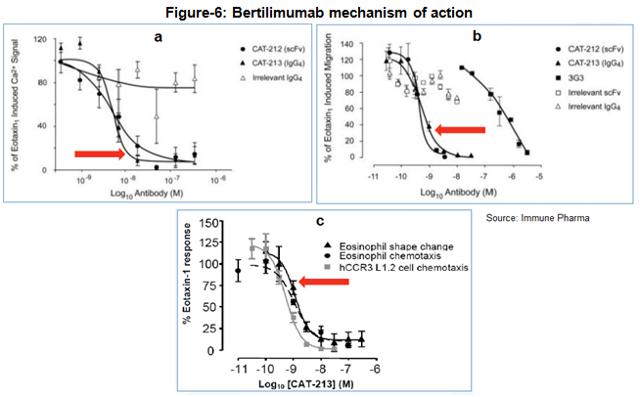
Multiple clinical and preclinical studies with bertilimumab confirm the drugs strong inhibition of eosinophil through the binding of eotaxin-1. For example, bertilimumab neutralizes the ability of eotaxin-1 to cause an increase in intracellular calcium signaling (Figure-6a), neutralizes eotaxin-1 mediated CCR3-expressing cell migration (Figure 6b), and inhibits eosinophil shape change and chemotaxis in vitro (Figure 6c) (source: Immune Pharma).
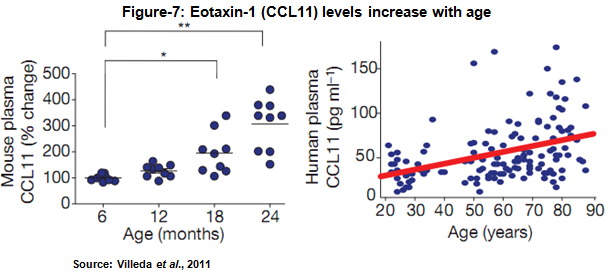
Pharmacokinetic data in 25 healthy male subjects found the elimination half-life to be approximately 14 days, consistent with other monoclonal antibodies. Safety data found no anti-bertilimumab antibody formation after a single intravenous dose. Beyond the blistering skin disease bullous pemphigoid, Immune Pharma sees the anti-eotaxin-1 mechanism offering therapeutic potential in other immuno-inflammatory disease such as Crohn’s disease and ulcerative colitis, atopic dermatitis, pulmonary and liver fibrosis, non-alcoholic fatty-liver disease and non-alcoholic steatohepatitis , and ovarian cancer. Scientists have even found increasing levels of eotaxin-1 correlate with age and decreased neurogenesis (Figure 7) (Villeda et al., 2011).
 Bertilimumab for the Treatment of BP
Bertilimumab for the Treatment of BP
Immune Pharma is currently conducting a Phase 2a open-label, proof-of-concept, single group study in adult patients with newly diagnosed, moderate to extensive BP (NCT0222146). The study will consist of three periods; a screening period of up to two weeks, an open-label treatment period lasting two weeks consisting of intravenous infusion of 10 mg/kg bertilimumab on Days 0 and 14, and a safety and efficacy follow-up period of approximately six weeks. Enrollment is slated for up to 15 patients and will take place at two centers in Israel. The first center, Sourasky-Ichilov Tel Aviv Medical Center in Tel Aviv, Israel is currently recruiting patients. A second center is expected to come online shortly.
Patients will receive concomitant low-dose oral prednisone starting at 30 mg per day during the treatment and follow-up period, which is approximately half of what a typical starting dose would be for a BP patient. The dosage of prednisone will be tapered down rapidly beginning as early as week one based on patient response. The primary goal of the study is to evaluate the safety and clinical efficacy of bertilimumab in newly diagnosed patients with BP. Key secondary objectives include evaluating additional efficacy and pharmacodynamic (PD) effects of the drug in this patient population. The primary efficacy analysis will be
disease control as measured by the Bullous Pemphigoid Disease Area Index (BPDAI), a quantitative measure of disease activity that takes into account the number and size of lesions as well as their location (skin vs. mucosa), as well as the percentage of patients who achieve a steroid dose of less than 10 mg per day. I anticipate initial data from the trial to be reported in late 2015 or early 2016.
Prior studies of bertilimumab have shown nearly a 100% reduction in eotaxin-1 levels within 24 hours of administering the drug, with more than 50% of the inhibition lasting for several weeks. Thus, while only dosing patients twice times (Days 0 and 14), the company believes that bertilimumab may provide enough of a rapid and sustained response to show initial proof-of-concept. Furthermore, I suspect that with the medium dose of steroids (30 mg prednisone per day) it is unlikely that more than 3 or 4 patients respond, thus potentially creating a low hurdle for bertilimumab to add value. As such, a successful outcome for the Phase 2a trial could be if only half of the targeted 15 patients respond to therapy with bertilimumab, as this would most likely be proof that the drug is working and can be advanced to the next clinical stage.
If the open-label Phase 2a study above is successful, Immune will likely push forward into a Phase 2/3 clinical trial in 2016. Because BP is an Orphan Disease, it seems logical to assume that this next trial will count as one of the required pivotal studies required prior to U.S. FDA approval. Immune has already filed for Orphan Drug designation for bertilimumab in BP in October 2014; however, the FDA responded saying they would like to see the data from the Phase 2a open-label study before they grant the designation. If all goes well, Orphan Drug designation could come during the first half of 2016. I see no reason why it should not be granted if the data are encouraging. As a reminder, Immune is working closely with medical opinion leaders and with the International Pemphigus and Pemphigoid Association (IPPF) to help develop better awareness of the disease and to accelerate the availability of new drugs to patients who need them.
A Quick Review of Atopic Dermatitis
Atopic Dermatitis (AD), also known as eczema, is a chronic inflammatory disease that results in itchy, red, swollen, and cracked skin. The itching and scratching typically leads to redness, swelling, cracking, “weeping” clear fluid, and finally, crusting and scaling. Unlike contact dermatitis or dyshidrotic eczema, atopic dermatitis is often inherited or associated with other systemic allergic reactions. However, similar to many other inflammatory skin diseases such as bullous pemphigoid and psoriasis, there are periods of exacerbations or flares and periods of remission.
The cause of AD is not known, but the disease seems to result from a combination of genetic (hereditary), immune system dysfunction, and environmental factors. Children are more likely to develop this disorder if a parent has had it or another atopic disease like asthma or hay fever. As some children with atopic dermatitis grow older, their skin disease improves or disappears altogether, although their skin often remains dry and easily irritated. In others, atopic dermatitis continues to be a significant problem into adulthood.
Treatment involves avoiding triggering factors, daily bathing with application of a moisturizing cream afterwards, and medications to help with itchiness (Tollefson et al., 2014). Regular, liberal use of emollients is recommended. The primary pharmacologic treatment is topical corticosteroids, which have been shown to reduce relapse rates in patients who have recurrent moderate to severe atopic dermatitis (Berke et al., 2012). Pimecrolimus and tacrolimus are calcineurin inhibitors that are recommended as second-line treatment for persons with moderate to severe atopic dermatitis and who are at risk of atrophy from topical corticosteroids, although the U.S. FDA has issued a boxed warning about a possible link between these medications and skin malignancies and lymphoma. Topical and oral antibiotics may be used to treat secondary bacterial infections, but are not effective in preventing atopic dermatitis flare-ups. The effectiveness of alternative therapies, such as Chinese herbal preparations, homeopathy, hypnotherapy/biofeedback, and massage therapy, has not been established (American Academy of Family Physicians, 2012).
According to the National Eczema Association, a substantial proportion of the U.S. population has either eczema – 31.6 million, or moderate to severe atopic dermatitis – 17.8 million. The prevalence of childhood eczema/atopic dermatitis in the U.S. is 10.7% overall and as high as 18.1% in individual states. Approximately one out of every three children with atopic dermatitis has moderate to severe disease. A recent study found that the prevalence of eczema in adults is 10.2%, which suggests that most children with eczema/atopic dermatitis continue to be affected even in adulthood. Three percent of U.S. adults have moderate to severe eczema/atopic dermatitis requiring systemic therapy. These numbers are much higher than for psoriasis, a disease that now has many good-targeted treatments for moderate to severe patients. Yet, there are still very large unmet needs for the treatment of patients with atopic dermatitis.
Bertilimumab for the Treatment of AD
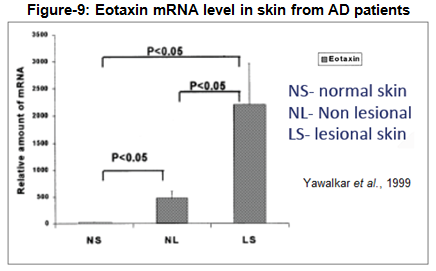
Chemokines are thought to play an important part in the development of inflammation in atopic dermatitis (Yawalkar et al., 1999). A paper published in the Journal of Investigative Dermatology (1999) showed that skin biopsy specimens obtained from non-lesional and lesional skin of patients with atopic dermatitis had significantly increased levels of eotaxin and CCR3 compared to non-atopic controls. As mentioned previously, CCR3 is known to be expressed by hematopoietic cells but some publications suggest CCR3 to be expressed by keratinocytes and fibroblasts, the latter suggesting a critical link to the pathogenesis of atopic dermatitis (Huber MA et al., 2002). Non-lesional atopic dermatitis CCR3 expression was also significantly increased at the mRNA and protein level, whereas eotaxin was increased at the mRNA level only. No significant difference in the expression of other chemoattractant activators of human eosinophil such as MCP-3, MIP-1alpha, and interleukin-8 was found in skin biopsy specimens from AD patients. The authors concluded that the enhanced local production of eotaxin may lead to the recruitment of eosinophils and T lymphocytes, which both express CCR3 and contribute to the initiation and maintenance of inflammation in patients with atopic dermatitis.
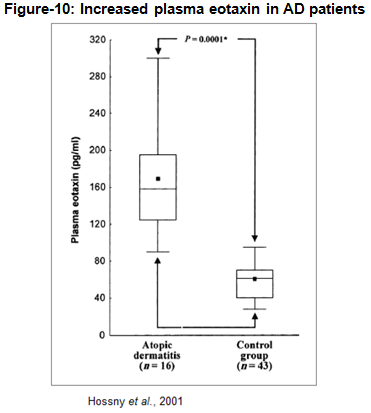
A paper published in Allergy (2001) investigated the level of circulating eotaxin in children with skin allergy in relation to clinical activity and lesion type. Plasma eotaxin was assayed in 78 infants and children, of whom 16 had AD, 19 had acute urticaria (AU), and 43 were healthy matched subjects. Results showed that the plasma eotaxin levels in children with AD (168±61 pg/ml) were significantly higher than the healthy control values (59.5±18.5 pg/ml) or in children with AU (124±33 pg/ml). The authors concluded that circulating levels of eotaxin increase in AD and during flares of AU, probably to serve in the recruitment and activation of eosinophils, and this may represent a biomarker of lesional activity (Hossny et al., 2001).
Immune Pharma plans to initiate clinical studies with bertilimumab in patients with atopic dermatitis in 2016. We believe this could be a significant label expansion for synergistic promotion for the drug when targeting dermatologists initially focusing on the BP indication. We note that Immune has also recently in-licensed a topical nano-formulation of Cyclosporin A in June 2015. Cyclosporin A is a commonly-used immunosuppressive agent in organ transplant patients to prevent rejection; however, the drug also offers utility in other immune-mediated conditions including psoriasis and atopic dermatitis. Unfortunately, there are limitations to administering the drug systemically, including variation in bioavailability, drug-drug interactions, and the potential for serious side effects including hypertension and nephrotoxicity. Therefore Immune pharma is planning to conduct a study in severe and/or refractory patients using bertilimumab.
Immune believes a topical formulation of Cyclosporin A should result in fewer adverse events due to the lack of systemic absorption. In a model of human skin, Immune’s topical cyclosporine A demonstrated comparable activity to the most potent topical corticosteroid, clobetasol. The company is planning to develop this topical nano-formulated version as a treatment for both mild psoriasis and mild to moderate atopic dermatitis. Similar to the planned label expansion of bertilimumab from BP to AD, this provides for leverage of a dermatology-focused sales force and synergy on the marketing and promotion side.
Conclusion
A quick search of the U.S. National Library of Medicine, part of the National Institutes of Health (“PubMed”) shows over 1,500 publications citing eotaxin-1. One of the most powerful pieces in support of eotaxin-1 as a therapeutic target can be seen in Figure 11 below. The authors, part of the Department of Immunodermatology and Allergology, Instituto Dermopatico dell’ Immacolata-IRCCS, Rome, Italy and as far as I can tell unaffiliated with Immune Pharmaceuticals, call eotaxin-1, “The most important cytokine involved in tissue inflammation.” Other peer-reviewed literature suggests eotaxin-1 sits at the crossroad of immunology. Immune’s bertilimumab, a potent and selective inhibitor of eotaxin-1, is the only active clinical-stage therapeutic that specifically targets this master regulator of inflammation. Quite interestingly, it is not an immunosuppressive drug, it is a patient-tailored immune regulatory therapy.

It is worth noting that with Bertilimumab and topical nano-cyclosporine A, Immune could become a major player in Immuno-Dermatology. Bertilimumab has potential in both bullous pemphigoid and severe atopic dermatitis. In the latter disease, it appears to be the only clinical stage biologic competing with Regeneron/Sanofi’s Phase 3, dupilumab, a monoclonal antibody against IL-4 receptor alpha subunit, which blocks signaling from both IL-4 and IL-13. IL-4 and IL-13 are key cytokines that are required for the initiation and maintenance of the Th2 (Type 2 helper T-cell) immune response that is believed to be a critical pathway in allergic inflammation. Topical nano-cyclosporine A is one of a handful of novel treatments for patients with moderate atopic dermatitis and psoriasis, along with crisaborole (AN2728), a PDE-4 inhibitor which recently successfully Phase 3 in atopic dermatitis and is developed by Anacor.
There are an estimated 65,000 patients in the U.S. and Europe with BP. The literature suggests the large majority of these patients can be treated safety with short-term immuno-suppressants and anti-inflammatory medications. However, in at least 25% of the acute BP patients long-term high-dose systemic corticosteroids are required, resulting in significant safety and tolerability issues for patients. Other patients are simply refractory to high dose steroids and require new treatment options. I suspect that bertilimumab, with mid-range biologic pricing similar to the anti-TNF molecules like Humira®, Remicade®, and Enbrel® use for the treatment of psoriasis (note I am not assuming orphan biologic pricing), the market opportunity for Immune in BP easily looks like $350 to $400 million. Atopic dermatitis, a condition that affects over 100 million globally, could easily double this peak potential opportunity for Immune or its commercial partner. Top-line data from the company’s Phase 2a BP study are expected late 2015 / early 2016.


