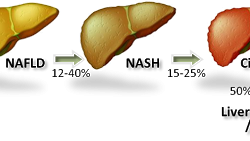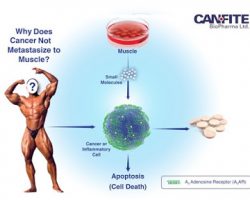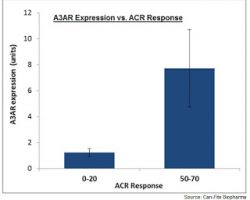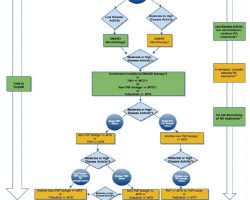
Can-Fite Phase 3 RA Program Ready To Start
Can-Fite Biopharma Ltd (NYSEMKT: CANF) is preparing to initiate a Phase 3 study with piclidenoson for the treatment of rheumatoid arthritis (RA) in the second quarter 2017. The Phase 3 study, called ACRobat, will investigate piclidenoson as a first line therapy and potential replacement for the current standard of care, methotrexate (MTX), in newly diagnosed…