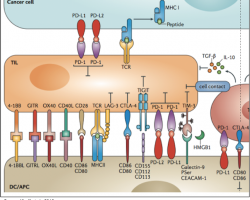
A Look At Cancer Immunotherapy And Some Key Players In 2017
Cancer immunotherapy, exploiting the abilities of the immune system to treat cancer, is fast becoming the standard of care for many malignancies and offers the best hope for curing patients of their disease. For biotech investors, the prospect of curing cancer has led to an abundance of opportunities as more and more companies enter the…










