Immunotherapy is intended to train and harness the body’s own immune system in order to recognize and target disease. Many approved immunotherapies are quickly becoming physicians best hope in the battle against cancer. While chemotherapy was once thought to be a breakthrough, immunotherapies have come to the forefront due to the targeted efficacy and less systemic side effects of these powerful medications.
One company pioneering new breakthroughs in cancer immunotherapy is Vaxil Bio Ltd (VXL.V). Vaxil was founded by Weizmann Institute scientists, one of Israel’s top research universities, in order to provide an altogether novel approach to cancer immunotherapy. The company has diligently advanced its immunotherapy platform and published its research and clinical results in top medical journals. Below is an introduction to Vaxil, its VaxHit™ technology, and ImMucin™, the company’s lead pipeline candidate for hematological malignancies and solid epithelial tumors.
Vaxil is an immunotherapy company developing a pipeline of novel therapeutic vaccines and antibodies for the treatment of cancer. The company’s lead development candidate is ImMucin™, a therapeutic cancer vaccine comprised of the signal peptide domain of the MUC1 tumor-associated antigen (TAA). Vaxil has shown that ImMucin possesses a high density of T- and B-cell epitopes while preserving MUC1 specificity (1). MUC1 (mucin 1) is a cell surface glycoprotein that is highly expressed by carcinomas and hematological tumors, including multiple myeloma (2). Recently, Vaxil has shown that ImMucin can potentially have strong anti-tumor activity when used in combination with checkpoint inhibitors. Additionally, ImMucin represents a new class of neoantigens, with the potential to strongly stimulate the body’s immune system (3).
Vaxil has completed an initial Phase 1/2 clinical trial (n=15) with ImMucin demonstrating the vaccine to be safe and highly tolerable in patients with multiple myeloma (MM). A robust immune response was seen in all patients treated with ImMucin, with the majority of patients achieving stable disease (4). A Phase 2 program is expected to commence in 2017 with ImMucin in a selected population of multiple myeloma patients. ImMucin was granted an Orphan Drug status for use in Multiple Myeloma in the U.S and the EU in 2015
VaxHit™ Platform Technology
ImMucin was discovered using Vaxil’s proprietary VaxHit platform technology. VaxHit combines algorithms which enable in-silico identification of signal peptides domains and their subsequent use as immunotherapeutic products. It is essentially a launchpad for unique and specific targeted immunotherapy products. VaxHit provides the ability to identify and select sequences within existing antigens with the potential to elicit a response in T-Cells, including CD4+ helper T-cells and CD8+ Killer T-cells, and in B-cell producing antibodies.

Importantly, during the antigen selection process, Vaxil scientists look for antigens that are highly expressed on tumor cells but less so on healthy cells.. The immune activation by the selected peptide sequence should be robust and impact both cellular and humoral immunity.. The use of signal peptides hold the promise of creating “universal” immunotherapies that target multiple patient populations and are less restricted to certain HLA subtypes.
Target Selection – MUC1
The target antigen for Vaxil’s lead development program, ImMucin, is MUC1. MUC1 is an oncogenic transmembrane glycoprotein in the mucin family found to be overexpressed in a wide variety of hematologic malignancies and solid tumors. For example, it is overexpressed in more than 90% of breast cancers (5) with clear differentiation between normal and malignant cells (6). Work out of Dana-Farber Cancer Institute / Harvard Medical School shows that blocking MUC1 induces death in breast cancer cells in vitro and in xenograft models (7). Implications of MUC1 aberrant expression have also been linked to lung cancers (8) and multiple myeloma (9).
With respect to multiple myeloma (MM), researchers out of Dana-Farber / Harvard demonstrated that MUC1 silencing by CRISPR editing results in downregulation of MYC messenger RNA and protein. The MYC oncoprotein is critical for MM cell and primary tumor survival. Studies further show that MUC1 levels positively correlate with MM progression and in primary cells from over 800 multiple myeloma patients. The findings are published in Blood (May 2016) and collectively provide convincing evidence that MUC1-C drives MYC expression in MM (10).
 ImMucin™ – A Novel Therapeutic Vaccine Targeting MUC1
ImMucin™ – A Novel Therapeutic Vaccine Targeting MUC1
ImMucin is a 21-mer cancer vaccine encoding the signal peptide domain of the MUC1 tumor-associated antigen and possesses a high density of T- and B-cell epitopes while preserving MUC1 specificity (11). ImMucin displays several potential advantages exclusive to synthetic peptide vaccines, including the induction of a highly specific immune response with negligible short-term toxicity, chemical stability, the absence of contaminants prevailing in recombinant products, and cost effective production and use which allows for a long-term therapeutic applications (12).
– ImMucin Mechanism of Action
ImMucin is a therapeutic cancer vaccine that targets a known tumor-associated antigen (TAA) in MUC1. This is a validated strategy and MUC1 has been well documented in the literature to be highly correlated with tumor progression (13, 14). Research out of the Universidad Nacional de La Plata, Argentina, shows that MUC1 copy number increases from normal tissue to primary invasive breast carcinomas in correlation with MUC1 protein expression (15). Unlike previous failed monoclonal antibodies that targeted the highly immunogenic extracellular tandem repeat array (TRA) of MUC1, ImMucin targets the signal peptide domain expressed on the cell surface with minimal MHC restriction.
In fact, ImMucin shows promiscuous binding properties to both MHC class I and class II alleles. Work published in Vaccine in 2011 shows ImMucin offers broad HLA binding and robust antigen-specific activation of human CD4+ and CD8+ T-cells obtained from healthy volunteers and cancer patients bearing various MUC1 positive tumors (16). In this study, strong anti-tumor activity was demonstrated in BALB/c mice vaccinated with MUC1 signal peptide targeting drug when dosed with GM-CSF vs. targeting MUC1-TRA or GM-CSF alone.
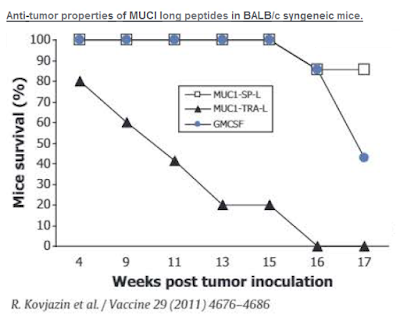 – ImMucin Phase 1/2 Study
– ImMucin Phase 1/2 Study
Vaxil Bio has completed a Phase 1/2 clinical study with ImMucin designed to assess the safety, immunity, and clinical response to 6 or 12 bi-weekly intradermal vaccinations, co-administered with human granulocyte macrophage colony-stimulating factor (GM-CSF) in 15 MUC1-positive multiple myeloma (MM) patients with residual or biochemically progressive disease following autologous stem cell transplantation. Results are published in the British Journal of Haematology (17).
The data show ImMucin to be well tolerated, with all adverse events temporal grade 1 or 2 and spontaneously resolved. ImMucin vaccination induced a robust increase in IFN-c-producing CD4+ and CD8+ T-cells, a pronounced population multimer CD8+ T-cells, a 94-fold increase in peripheral blood mononuclear cells proliferation and 7-fold increase in anti-ImMucin antibodies, accompanied with T-cell and antibody-dependent cell mediated cytotoxicity.
– ImMucin Phase 1/2 Study
Vaxil Bio has completed a Phase 1/2 clinical study with ImMucin designed to assess the safety, immunity, and clinical response to 6 or 12 bi-weekly intradermal vaccinations, co-administered with human granulocyte macrophage colony-stimulating factor (GM-CSF) in 15 MUC1-positive multiple myeloma (MM) patients with residual or biochemically progressive disease following autologous stem cell transplantation. Results are published in the British Journal of Haematology (18).
The data show ImMucin to be well tolerated, with all adverse events temporal grade 1 or 2 and spontaneously resolved. ImMucin vaccination induced a robust increase in IFN-c-producing CD4+ and CD8+ T-cells, a pronounced population multimer CD8+ T-cells, a 94-fold increase in peripheral blood mononuclear cells proliferation and 6-8-fold increase in anti-ImMucin antibodies, accompanied with T-cell and antibody-dependent cell mediated cytotoxicity.
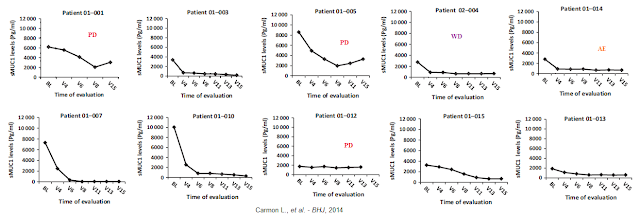
A significant decrease in soluble MUC1 levels was observed in 9/10 patients. The importance of this result lies in the presumed correlation between disease progression and soluble MUC1 levels in the circulation. It is therefore anticipated that this parameter will serve in future clinical trials and in the clinic to evaluate the effect of ImMucin in reducing disease burden during treatment. A significant decrease in soluble MUC1 levels was observed in 9/10 patients.
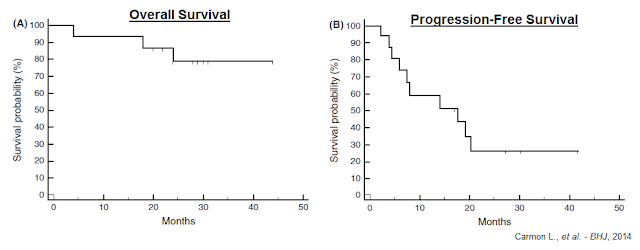
Stable disease or improvement, persisting for 17.5-41.3 months (ongoing at the time of publication) was achieved in 11/15 patients and appeared to be associated with low-intermediate PDL1 (CD274) bone marrow levels pre- and post-vaccination. Below is the overall survival (A) and progression-free survival (B) for the entire group of vaccinated patients, starting from initial vaccination.
These Phase 1/2 data show ImMucin is a highly tolerable cancer vaccine that induces robust and diversified T- and B-cell ImMucin specific immunity in late-stage multiple myeloma patients across major histocompatibility complex barrier, resulting in at least disease stabilization in most patients.
What’s On Deck For Vaxil & ImMucin in 2017
Late last month, Vaxil Bio announced that it was seeing encouraging preliminary pre-clinical data in testing ImMucin in combination with checkpoint inhibitors (see press release). Immune checkpoints are proteins that distinguish normal healthy cells from foreign or cancerous cells and act as negative regulators of the immune system. Immune checkpoints play critical roles in maintaining self-tolerance, preventing autoimmunity and protecting tissues from immune collateral damage. Certain immune checkpoints need to be activated (or inactivated) in order for the body to initiate an immune response. Certain times, cancer cells find a way to use these checkpoints to avoid being attacked by the immune system.
PD-1 is a checkpoint protein on immune T-cells. It normally acts as a type of “off switch” that helps keep the T-cells from attacking other cells in the body. It does this when it attaches to PD-L1, a protein on some normal (and cancer) cells. When PD-1 binds to PD-L1, it basically tells the T-cell to leave the other cell alone. Some cancer cells have large amounts of PD-L1, which helps them evade immune attack. Monoclonal antibodies that inhibit PD-1, such as nivolumab (Opdivo®) and pembrolizumab (Keytruda®) boost the immune response against cancer cells and have shown a great deal of promise in treating certain cancers.
Vaxil Bio believes that targeting MUC1 is synergistic with the use of immune checkpoint inhibitors. The checkpoint inhibitors can turn off the tumors ability to mask itself among healthy issue and then ImMucin follows as a neoantigen to enhance specific T-cell and B-cell immune response to the tumor-associated MUC1 antigen.
Transgene is a Paris-based biopharma company developing a similar MUC1-targeting cancer vaccine. Transgene just recently announced the initiation of an investigator-sponsored clinical program at UC Davis that will combined TG4010 with Opdivo for the treatment of non-small cell lung cancer (see press release). This is not only a validation of the strategy to target MUC1 and combined with checkpoint inhibitors, but also suggests a second potential path forward for ImMucin in lung cancer beyond multiple myeloma.
Management also believes that ImMucin may have use in CAR-T therapeutics. In short, CAR-T is the process of retraining the body’s own immune system to find and attack cancerous cells. In CAR-T, T-cells are genetically engineered (transfected) through the use of a virus to express a receptor for certain antigens, such as CD19 (JUNO, NVS) or BCMA (BLUE), found on both healthy and malignant B cells. Once returned to the patient, these engineered T-cells actively find and kill B cells, malignant or healthy, to eliminate cancer from the body. Recently, Bluebird Bio announced impressive early-stage data testing its new BCMA-targeting CAR-T therapy in patients with multiple myeloma. The ImMucin Phase 1/2 data noted above was in patients with multiple myeloma. By using MUC1 specific therapies such as ImMucin novel CAR-Ts may be designed., Vaxil Bio is hoping to create a new breakthrough therapy with this approach.
I believe combination therapy is the future for multiple myeloma treatment, and the Bluebird Bio BCMA data at ASH was quite impressive for such an early-stage program. The competitive bar in multiple myeloma is quite high, with leading therapies like Revlimid® (CELG) and Kyprolis® (AMGN) likely to give way to new immunotherapies like CAR-T. Drugs like Opdivo® and Keytruda® likely also have their place, and the opportunity to safely combined PD-1 targeting mAbs with therapeutic cancer vaccines like ImMucin holds tremendous promise.
Conclusion
Therapeutic cancer vaccines have an abysmal history, and with the exception Provenge® (sipuleucel-T), an ex vivo dendritic cell vaccine for prostate cancer, no therapeutic cancer vaccine has yet shown clinical efficacy in a Phase 3 randomized trial. I expect that to change in the near future thanks to improvements in transcriptome sequencing and computational modeling platforms.
Vaxil’s VaxHit platform technology combines proprietary algorithms which enable in silico identification of signal peptides domains and their subsequent use as immunotherapeutic products. Using VaxHit, the company has identified ImMucin, a signal peptide domain of the MUC1 tumor-associated antigen, as an interesting candidate for clinical development. And, unlikely previous biopharma efforts to target MUC1 that focused on the highly immunogenic extracellular tandem repeat array, ImMucin targets the signal peptide domain expressed on the cell surface with minimal MHC restriction.
Initial clinical data with ImMucin has been encouraging, and recently the company noted its intention to study the vaccine in combination with other immunotherapies, including checkpoint inhibitors and CAR-T therapeutics. I believe this presents the company with the potential opportunity to form research collaborations with larger biopharma organizations (e.g. CELG, AMGN, NVS, JNJ) developing these candidates for the treatment of multiple myeloma.
This is an area where investors have seen several deals between big pharma players and smaller organizations with drug candidates similar in stage to Vaxil’s ImMucin. Vaxil’s market value is only $6 million as of today. The average upfront payment or equity investment from the three representative deals shown below range between 10 and 30x Vaxil’s current value.
Multiple myeloma is an Orphan disease in the U.S., affecting roughly 33,000 individuals in the U.S. each year according to the American Cancer Society. ImMucin is expected to qualify as an Orphan Drug in the U.S., which will provide certain cost and time advantages to development, as well as market exclusivity one approved. I expect the company to move into Phase 2 in 2017, which should start the process of unlocking tremendous value for investors in VXL.V / VXLLF shares.


